 Found 15 hits Enzyme Inhibition Constant Data
Found 15 hits Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Competitive inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using varying levels of L-arginine-7-amino-4-M... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Inhibition of human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H using L-arginine-7-amino-4-Methylcoumarine as substrate pr... |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
ENSCMu
| Assay Description
Spectrophotometric assays were performed by monitoring hydrolysis of chromogenic substrate. The release of para-nitroaniline at 405 nm was measured t... |
Bioorg Med Chem 14: 7241-57 (2006)
Article DOI: 10.1016/j.bmc.2006.06.050
BindingDB Entry DOI: 10.7270/Q2N29V73 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoire de Chimie Organique et Bioorganique, FRE3252, ENSCMu, F-68093 Mulhouse Cedex, France.
Curated by ChEMBL
| Assay Description
Inhibition of aminopeptidase activity of human leukotriene A4 hydrolase by Dixon plot analysis |
Bioorg Med Chem 19: 1434-49 (2011)
Article DOI: 10.1016/j.bmc.2011.01.008
BindingDB Entry DOI: 10.7270/Q2G44QJQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Santen Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant leukotriene A4 hydrolase |
Bioorg Med Chem Lett 18: 4529-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.043
BindingDB Entry DOI: 10.7270/Q2PR7VSQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against Leukotriene A4 hydrolase from human leukocytes |
Bioorg Med Chem Lett 1: 551-556 (1991)
Article DOI: 10.1016/S0960-894X(01)80464-9
BindingDB Entry DOI: 10.7270/Q2M32W8B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University
Curated by ChEMBL
| Assay Description
Inhibition of human LTA4H epoxide hydrolase activity using LTA4 as substrate incubated for 15 mins prior to substrate addition measured after 10 mins... |
Eur J Med Chem 59: 160-7 (2013)
Article DOI: 10.1016/j.ejmech.2012.10.057
BindingDB Entry DOI: 10.7270/Q27P90QH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mansoura University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His-tagged human LTA4H aminopeptidase activity expressed in Escherichia coli using L-alanine-4-nitro-anilide hyd... |
Bioorg Med Chem 25: 1277-1285 (2017)
Article DOI: 10.1016/j.bmc.2016.12.048
BindingDB Entry DOI: 10.7270/Q2SJ1NMK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Mansoura University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant C-terminal His-tagged human LTA4H epoxide hydrolase activity expressed in Escherichia coli assessed as reduction in LTB4 pr... |
Bioorg Med Chem 25: 1277-1285 (2017)
Article DOI: 10.1016/j.bmc.2016.12.048
BindingDB Entry DOI: 10.7270/Q2SJ1NMK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM50046325
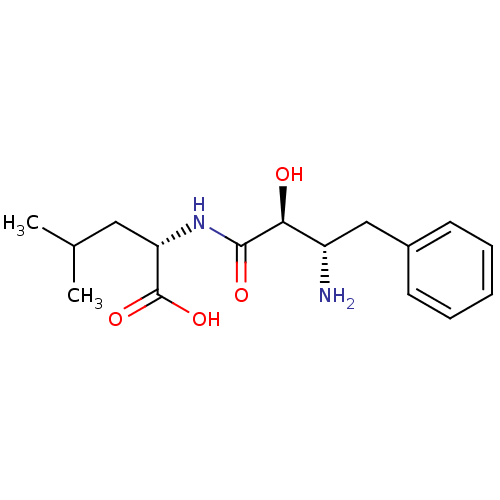
((S)-2-((2S,3S)-3-Amino-2-hydroxy-4-phenyl-butyryla...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of amidase activity of LTA4 hydrolase purified from human leukocytes |
J Med Chem 36: 211-20 (1993)
BindingDB Entry DOI: 10.7270/Q2T152P3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM50294170
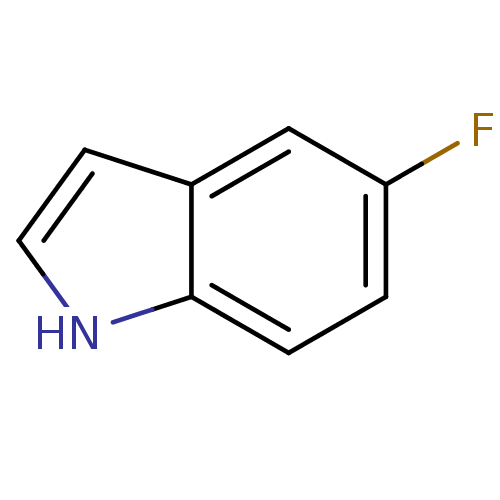
(5-fluoro-1H-indole | CHEMBL555457)Show InChI InChI=1S/C8H6FN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
Article
PubMed
| n/a | n/a | >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of peptidase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE |
J Med Chem 52: 4694-715 (2009)
Article DOI: 10.1021/jm900259h
BindingDB Entry DOI: 10.7270/Q2F47P6N |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM50294170

(5-fluoro-1H-indole | CHEMBL555457)Show InChI InChI=1S/C8H6FN/c9-7-1-2-8-6(5-7)3-4-10-8/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
Article
PubMed
| n/a | n/a | >2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
deCODE biostructures, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of hydrolase activity of human recombinant LTA4H expressed in Escherichia coli BL21-AI/pRARE assessed as LTB4 formation by tandem quadrupo... |
J Med Chem 52: 4694-715 (2009)
Article DOI: 10.1021/jm900259h
BindingDB Entry DOI: 10.7270/Q2F47P6N |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 5.15E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 35 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 4.63E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 15 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(611/611 = 100%)†
(Homo sapiens (Human)) | BDBM23971

((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H24N2O4/c1-10(2)8-13(16(21)22)18-15(20)14(19)12(17)9-11-6-4-3-5-7-11/h3-7,10,12-14,19H,8-9,17H2,1-2H3,(H,18,20)(H,21,22)/t12-,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a |
Goethe University Frankfurt
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal his6-tagged/N-terminal T7 gene leader sequence-tagged LTA4H at 25 degC by ITC method |
Bioorg Med Chem 24: 5243-5248 (2016)
Article DOI: 10.1016/j.bmc.2016.08.047
BindingDB Entry DOI: 10.7270/Q2959KH9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data