

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510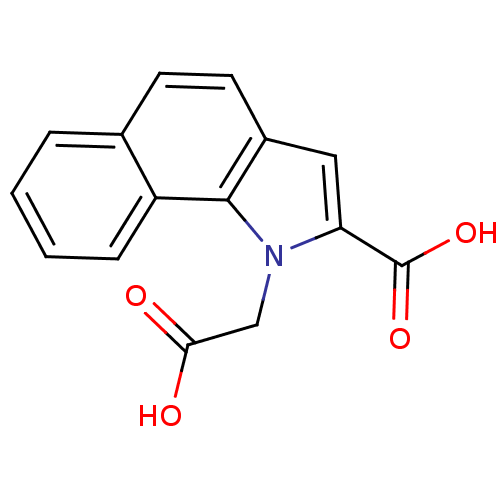 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.4 | 23 |
Novartis Institutes for Biomedical Research | Assay Description A biophysical assay using NMR spectroscopy to identify fragments with higher binding affinity to human recombinant FPPS. | Nat Chem Biol 6: 660-6 (2010) Article DOI: 10.1038/nchembio.421 BindingDB Entry DOI: 10.7270/Q2KP80HJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of FPPS (unknown origin) | J Med Chem 57: 5764-76 (2014) Article DOI: 10.1021/jm500629e BindingDB Entry DOI: 10.7270/Q2GH9KJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Chimie des Substances Naturelles Curated by ChEMBL | Assay Description Inhibition of FPPS (unknown origin) | Bioorg Med Chem 22: 4474-89 (2014) Article DOI: 10.1016/j.bmc.2014.04.019 BindingDB Entry DOI: 10.7270/Q29K4CW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of N-terminal His-tagged human recombinant FPPS expressed in Escherichia coli BL21 (DE3) using GPP and [3H]-IPP as substrate preincubated ... | J Med Chem 60: 2119-2134 (2017) Article DOI: 10.1021/acs.jmedchem.6b01888 BindingDB Entry DOI: 10.7270/Q23F4RXR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human FPPS | Bioorg Med Chem Lett 25: 1117-23 (2015) Article DOI: 10.1016/j.bmcl.2014.12.089 BindingDB Entry DOI: 10.7270/Q25X2BMF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... | J Med Chem 56: 7939-50 (2013) Article DOI: 10.1021/jm400946f BindingDB Entry DOI: 10.7270/Q20V8F6B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... | J Med Chem 57: 5764-76 (2014) Article DOI: 10.1021/jm500629e BindingDB Entry DOI: 10.7270/Q2GH9KJR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (348/348 = 100%)† (Homo sapiens (Human)) | BDBM36510 (1-(Carboxymethyl)-1H-benzo[g]indole-2-carboxylic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition by scintillation counting analy... | J Med Chem 56: 7939-50 (2013) Article DOI: 10.1021/jm400946f BindingDB Entry DOI: 10.7270/Q20V8F6B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||