 Found 11 hits Enzyme Inhibition Constant Data
Found 11 hits Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 8
(337/361 = 93%)†
(Homo sapiens (Human)) | BDBM16018
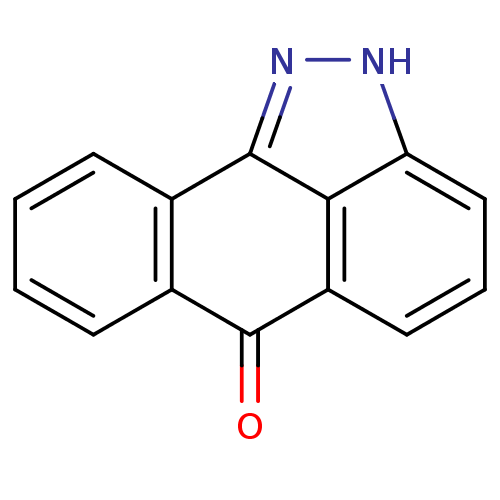
(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 |
J Med Chem 53: 3005-12 (2010)
Article DOI: 10.1021/jm9003279
BindingDB Entry DOI: 10.7270/Q2KS6RP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(313/361 = 87%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK2 |
J Med Chem 53: 3005-12 (2010)
Article DOI: 10.1021/jm9003279
BindingDB Entry DOI: 10.7270/Q2KS6RP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(337/361 = 93%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| |
ACS Chem Biol 8: 1747-54 (2013)
Article DOI: 10.1021/cb3006165
BindingDB Entry DOI: 10.7270/Q25719PN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(362/362 = 100%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 |
J Med Chem 53: 3005-12 (2010)
Article DOI: 10.1021/jm9003279
BindingDB Entry DOI: 10.7270/Q2KS6RP5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(337/361 = 93%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human JNK1 by radiometric assay |
Bioorg Med Chem 16: 4715-32 (2008)
Article DOI: 10.1016/j.bmc.2008.02.027
BindingDB Entry DOI: 10.7270/Q2JS9R8M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 9
(313/361 = 87%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(362/362 = 100%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Merck Research Laboratories
| Assay Description
HTRF relies on fluorescence resonance energy transfer (FRET) between the donor, a europium cryptate (EuK), and the acceptor, the light harvesting pro... |
Chem Biol 10: 705-12 (2003)
Article DOI: 10.1016/S1074-5521(03)00159-5
BindingDB Entry DOI: 10.7270/Q2DJ5CWZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(362/362 = 100%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
The binding results were confirmed by measuring IC50 for the inhibition of JNK kinase activity by using Z'-LYTE assay format. |
Chem Biol 19: 140-54 (2012)
Article DOI: 10.1016/j.chembiol.2011.11.010
BindingDB Entry DOI: 10.7270/Q2K35S8X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(362/362 = 100%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
| |
ACS Chem Biol 8: 1747-54 (2013)
Article DOI: 10.1021/cb3006165
BindingDB Entry DOI: 10.7270/Q25719PN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 10
(362/362 = 100%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hanyang University
Curated by ChEMBL
| Assay Description
Inhibition of JNK3 in human PBMCs assessed as decrease in LPS-induced TNFalpha mRNA level after 4 hrs by real-time reverse transcription-PCR analysis |
Bioorg Med Chem 21: 2271-85 (2013)
Article DOI: 10.1016/j.bmc.2013.02.021
BindingDB Entry DOI: 10.7270/Q29W0GVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 8
(337/361 = 93%)†
(Homo sapiens (Human)) | BDBM16018

(14,15-diazatetracyclo[7.6.1.0^{2,7}.0^{13,16}]hexa...)Show InChI InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Medical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of JNK1 using ATF2 substrate by TR-FRET assay |
J Med Chem 54: 6206-14 (2011)
Article DOI: 10.1021/jm200479c
BindingDB Entry DOI: 10.7270/Q2668DMM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data