 Found 8 hits Enzyme Inhibition Constant Data
Found 8 hits Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4A/4B/4C/4D
(285/333 = 86%)†
(Homo sapiens (Human)) | BDBM50085135
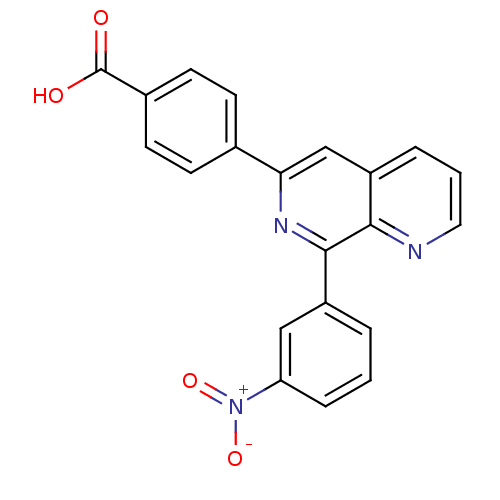
(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of [3H]rolipram binding in human peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(285/333 = 86%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4D from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(285/333 = 86%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Matrix Laboratories Limited
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D |
J Med Chem 51: 5471-89 (2008)
Article DOI: 10.1021/jm800582j
BindingDB Entry DOI: 10.7270/Q2GQ6ZNK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(285/333 = 86%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4D (PDE4D) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(333/333 = 100%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Glasgow
Curated by ChEMBL
| Assay Description
Inhibition of full-length PDE4A4 using cAMP as substrate by two-step radiochemical assay |
J Med Chem 54: 3331-47 (2011)
Article DOI: 10.1021/jm200070e
BindingDB Entry DOI: 10.7270/Q2CF9R75 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(333/333 = 100%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibitory activity against Phosphodiesterase 4A (PDE4A) from human source expressed in Saccharomyces cerevisiae |
J Med Chem 43: 675-82 (2000)
BindingDB Entry DOI: 10.7270/Q2N58N3G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(333/333 = 100%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4A from peripheral blood mononuclear cells |
Bioorg Med Chem Lett 12: 233-5 (2001)
BindingDB Entry DOI: 10.7270/Q2MG7Q1T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(333/333 = 100%)†
(Homo sapiens (Human)) | BDBM50085135

(4-(8-(3-nitrophenyl)-1,7-naphthyridin-6-yl)benzoic...)Show SMILES OC(=O)c1ccc(cc1)-c1cc2cccnc2c(n1)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C21H13N3O4/c25-21(26)14-8-6-13(7-9-14)18-12-16-4-2-10-22-19(16)20(23-18)15-3-1-5-17(11-15)24(27)28/h1-12H,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
University of Glasgow
Curated by ChEMBL
| Assay Description
Induction of SQSTM1-dependent intracellular redistribution of GFP-tagged PDE4A4 assessed as maximal accretion of enzyme into foci |
J Med Chem 54: 3331-47 (2011)
Article DOI: 10.1021/jm200070e
BindingDB Entry DOI: 10.7270/Q2CF9R75 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data