

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine receptor subunit delta (Torpedo californica) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Agonist activity against Nicotinic acetylcholine receptor (nAchR) | J Med Chem 42: 1481-500 (1999) Article DOI: 10.1021/jm9805034 BindingDB Entry DOI: 10.7270/Q2HX1DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50076327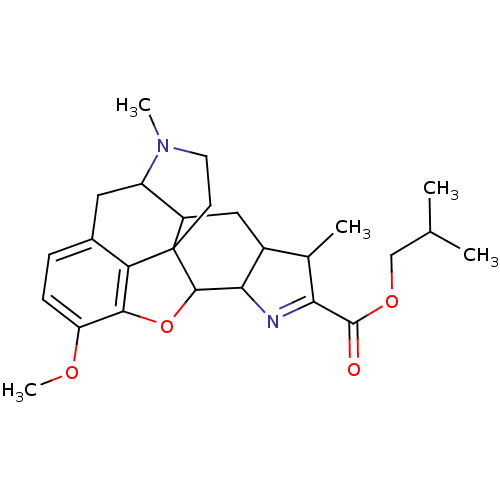 (CHEMBL34663 | isobutyl 12-methoxy-5,18-dimethyl-10...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Opioid receptor delta 1 agonist activity was determined | J Med Chem 42: 1481-500 (1999) Article DOI: 10.1021/jm9805034 BindingDB Entry DOI: 10.7270/Q2HX1DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2C (Rattus norvegicus (Rat)) | BDBM50062599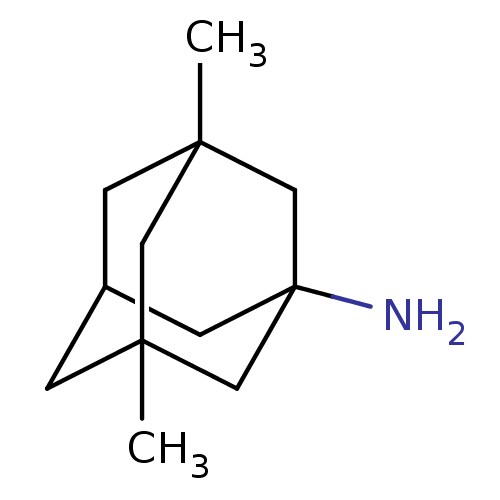 (3,5-Dimethyl-adamantan-1-ylamine | CHEMBL807 | EN3...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound with the N-methyl-D-aspartate glutamate receptor blocking activity | J Med Chem 42: 1481-500 (1999) Article DOI: 10.1021/jm9805034 BindingDB Entry DOI: 10.7270/Q2HX1DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50176259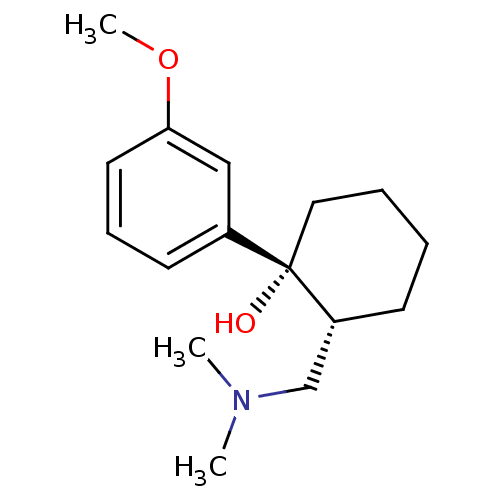 ((1R,2R)-2-[(dimethylamino)methyl]-1-(3-methoxyphen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Opioid receptor mu 1 agonist activity with monoamine (NE, 5-HT) uptake-blocking activity in the 0.8-1 uM range | J Med Chem 42: 1481-500 (1999) Article DOI: 10.1021/jm9805034 BindingDB Entry DOI: 10.7270/Q2HX1DCV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||