

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase TEM (Escherichia coli) | BDBM50076678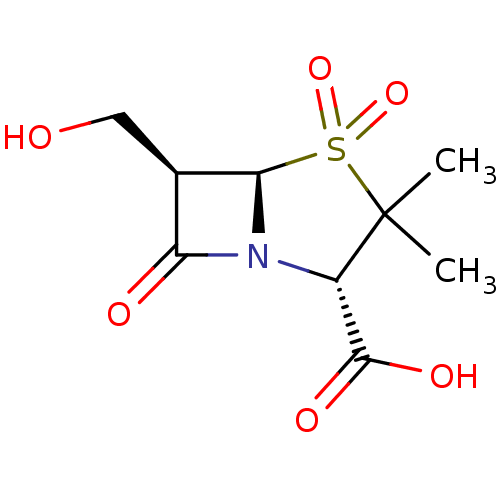 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50403733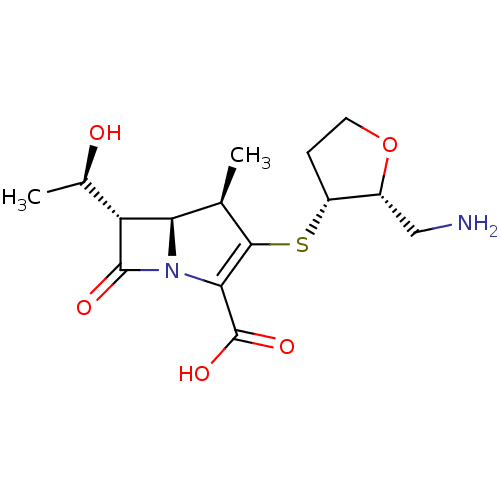 (CHEMBL1206880 | CL-191121) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50157692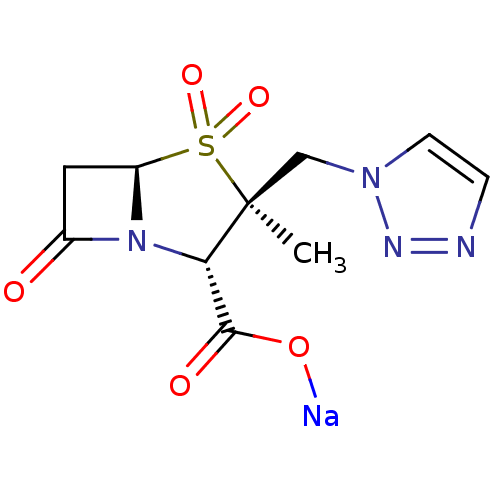 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076671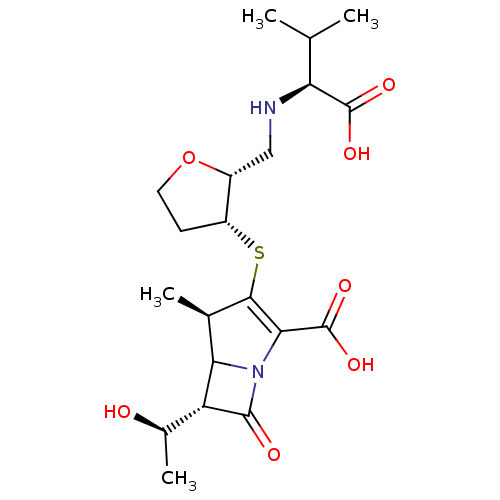 ((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076671 ((4R,6S)-3-{(2R,3R)-2-[((S)-1-Carboxy-2-methyl-prop...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076673 (CHEMBL175189 | Sodium; (2S,5R,6R)-6-((S)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50403733 (CHEMBL1206880 | CL-191121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076678 ((2S,5R,6R)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50021954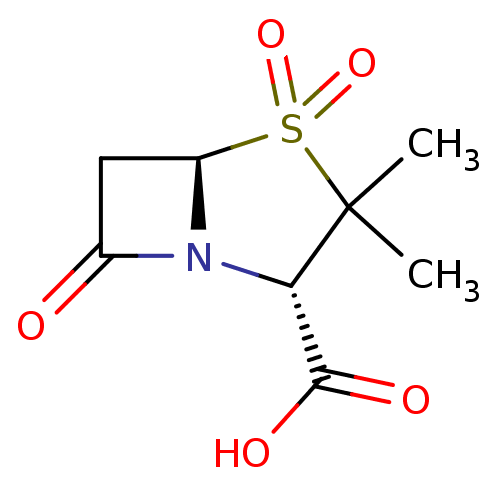 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076674 (CHEMBL177772 | Sodium; (2S,5R,6S)-6-((S)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076672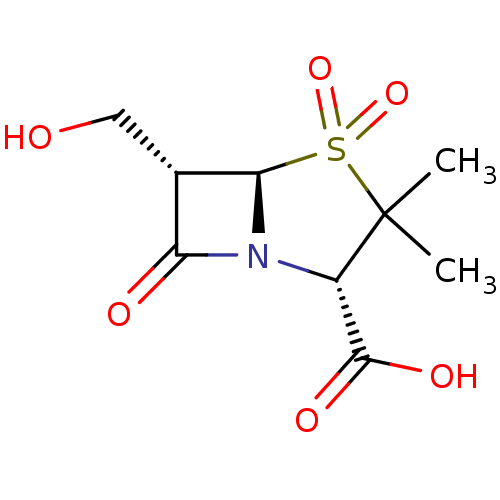 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076672 ((2S,5R,6S)-6-Hydroxymethyl-3,3-dimethyl-4,4,7-trio...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076677 (CHEMBL177912 | Sodium; (2S,5R,6S)-6-((R)-1-hydroxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076675 (CHEMBL265722 | Sodium; (2S,5R,6R)-6-hydroxymethyl-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50157692 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076677 (CHEMBL177912 | Sodium; (2S,5R,6S)-6-((R)-1-hydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076676 (CHEMBL177463 | Sodium; (2S,5R,6S)-6-hydroxymethyl-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50021954 ((2S,5R)-3,3-Dimethyl-4,4,7-trioxo-4lambda*6*-thia-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076675 (CHEMBL265722 | Sodium; (2S,5R,6R)-6-hydroxymethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076673 (CHEMBL175189 | Sodium; (2S,5R,6R)-6-((S)-1-hydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Escherichia coli) | BDBM50076676 (CHEMBL177463 | Sodium; (2S,5R,6S)-6-hydroxymethyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against AmpC (class C) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50076674 (CHEMBL177772 | Sodium; (2S,5R,6S)-6-((S)-1-hydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >3.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibitory activity of the compound against TEM-1 (class A) beta-lactamase | Bioorg Med Chem Lett 9: 991-6 (1999) BindingDB Entry DOI: 10.7270/Q2TB163Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||