 Found 68 hits of Enzyme Inhibition Constant Data
Found 68 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Angiotensin-converting enzyme
(Homo sapiens (Human)) | BDBM50369460
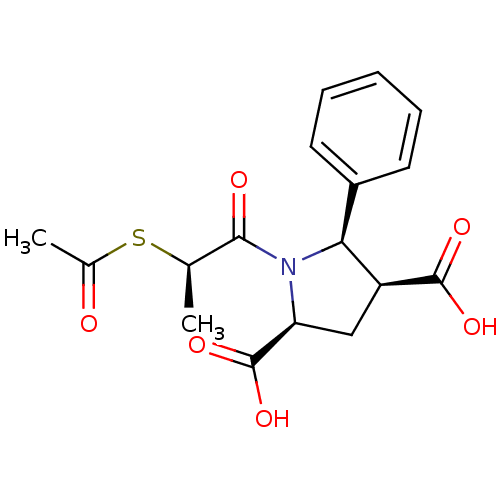
(CHEMBL1788109)Show SMILES C[C@@H](SC(C)=O)C(=O)N1[C@@H](C[C@@H]([C@@H]1c1ccccc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C17H19NO6S/c1-9(25-10(2)19)15(20)18-13(17(23)24)8-12(16(21)22)14(18)11-6-4-3-5-7-11/h3-7,9,12-14H,8H2,1-2H3,(H,21,22)(H,23,24)/t9-,12+,13+,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Enzyme inhibitory activity towards Angiotensin I converting enzyme |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM36187
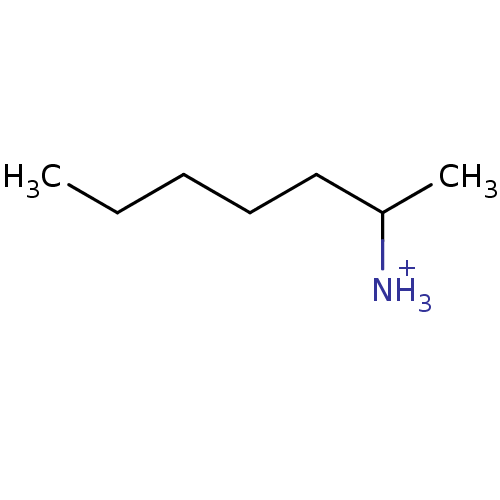
(1-methylhexylamine | Tuaminoheptane)Show InChI InChI=1S/C7H17N/c1-3-4-5-6-7(2)8/h7H,3-6,8H2,1-2H3/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Antagonistic activity towards Opioid receptor mu 1 |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271772

((3S,6S)-7-(3-Cyclohexyl-propyl)-6-(4-methoxy-benzy...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)C[NH+]=C3N2CCCC2CCCCC2)cc1 |r,c:13| Show InChI InChI=1S/C23H35N3O/c1-18-16-24-23-25(14-6-9-19-7-4-3-5-8-19)21(17-26(18)23)15-20-10-12-22(27-2)13-11-20/h10-13,18-19,21H,3-9,14-17H2,1-2H3/p+1/t18-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50369456

(5,6,7,8-TETRAHYDROISOQUINOLINE)Show InChI InChI=1S/C9H11N/c1-2-4-9-7-10-6-5-8(9)3-1/h5-7H,1-4H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Sigma opioid receptor type 1 |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM7837
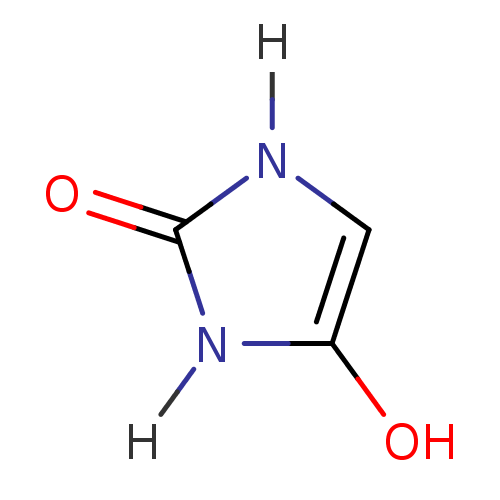
(CHEMBL122334 | Hydantoin Derivative 36 | imidazoli...)Show InChI InChI=1S/C3H4N2O2/c6-2-1-4-3(7)5-2/h1,6H,(H2,4,5,7) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Sigma opioid receptor type 1 |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271773

((3S,6R)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-6-(4-m...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C)C[NH+]=C3N2CCC2CC3CCC2C3)cc1 |r,c:13,THB:17:18:24:22.21| Show InChI InChI=1S/C23H33N3O/c1-16-14-24-23-25(10-9-20-12-18-3-6-19(20)11-18)21(15-26(16)23)13-17-4-7-22(27-2)8-5-17/h4-5,7-8,16,18-21H,3,6,9-15H2,1-2H3/p+1/t16-,18?,19?,20?,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271816
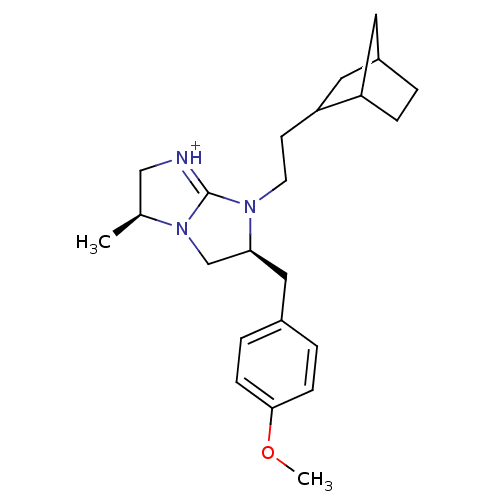
((3S,6S)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-6-(4-m...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)C[NH+]=C3N2CCC2CC3CCC2C3)cc1 |r,c:13,THB:17:18:24:22.21| Show InChI InChI=1S/C23H33N3O/c1-16-14-24-23-25(10-9-20-12-18-3-6-19(20)11-18)21(15-26(16)23)13-17-4-7-22(27-2)8-5-17/h4-5,7-8,16,18-21H,3,6,9-15H2,1-2H3/p+1/t16-,18?,19?,20?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271818
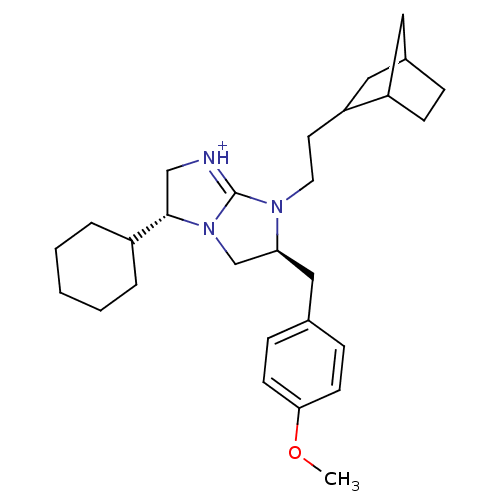
((3R,6S)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-3-cycl...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C[NH+]=C3N2CCC2CC3CCC2C3)C2CCCCC2)cc1 |r,c:12,THB:16:17:23:21.20| Show InChI InChI=1S/C28H41N3O/c1-32-26-11-8-20(9-12-26)17-25-19-31-27(22-5-3-2-4-6-22)18-29-28(31)30(25)14-13-24-16-21-7-10-23(24)15-21/h8-9,11-12,21-25,27H,2-7,10,13-19H2,1H3/p+1/t21?,23?,24?,25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 219 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271869
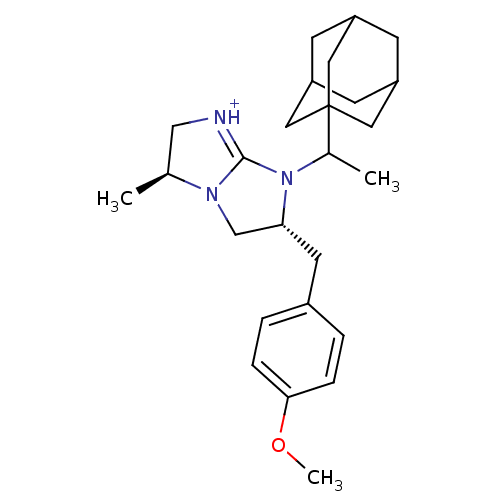
((3S,6R)-7-(1-Adamantan-1-yl-ethyl)-6-(4-methoxy-be...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C)C[NH+]=C3N2C(C)C23CC4CC(CC(C4)C2)C3)cc1 |r,c:13,TLB:16:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18| Show InChI InChI=1S/C26H37N3O/c1-17-15-27-25-28(17)16-23(11-19-4-6-24(30-3)7-5-19)29(25)18(2)26-12-20-8-21(13-26)10-22(9-20)14-26/h4-7,17-18,20-23H,8-16H2,1-3H3/p+1/t17-,18?,20?,21?,22?,23+,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 238 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271871
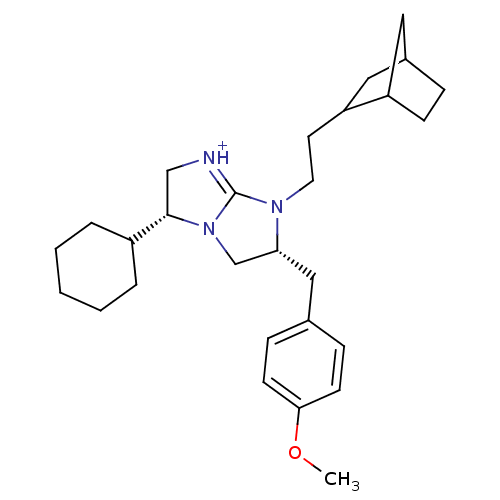
((3R,6R)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-3-cycl...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C[NH+]=C3N2CCC2CC3CCC2C3)C2CCCCC2)cc1 |r,c:12,THB:16:17:23:21.20| Show InChI InChI=1S/C28H41N3O/c1-32-26-11-8-20(9-12-26)17-25-19-31-27(22-5-3-2-4-6-22)18-29-28(31)30(25)14-13-24-16-21-7-10-23(24)15-21/h8-9,11-12,21-25,27H,2-7,10,13-19H2,1H3/p+1/t21?,23?,24?,25-,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271916
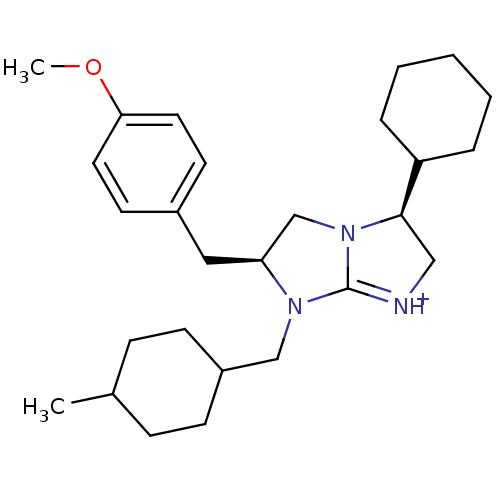
((3S,6S)-3-Cyclohexyl-6-(4-methoxy-benzyl)-7-(4-met...)Show SMILES COc1ccc(C[C@H]2CN3[C@H](C[NH+]=C3N2CC2CCC(C)CC2)C2CCCCC2)cc1 |r,wD:10.25,7.6,c:12,(.94,-35.88,;1.67,-37.25,;.87,-38.56,;1.6,-39.93,;.79,-41.23,;-.75,-41.18,;-1.56,-42.49,;-3.1,-42.46,;-3.97,-41.19,;-5.45,-41.62,;-6.91,-41.11,;-7.84,-42.33,;-6.98,-43.6,;-5.49,-43.17,;-4.04,-43.69,;-3.6,-45.16,;-2.1,-45.52,;-1.66,-46.99,;-.17,-47.35,;.89,-46.24,;2.39,-46.6,;.46,-44.76,;-1.04,-44.39,;-7.34,-39.63,;-6.28,-38.52,;-6.72,-37.05,;-8.21,-36.68,;-9.28,-37.8,;-8.84,-39.28,;-1.49,-39.83,;-.68,-38.52,)| Show InChI InChI=1S/C27H41N3O/c1-20-8-10-22(11-9-20)18-29-24(16-21-12-14-25(31-2)15-13-21)19-30-26(17-28-27(29)30)23-6-4-3-5-7-23/h12-15,20,22-24,26H,3-11,16-19H2,1-2H3/p+1/t20?,22?,24-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 336 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271917
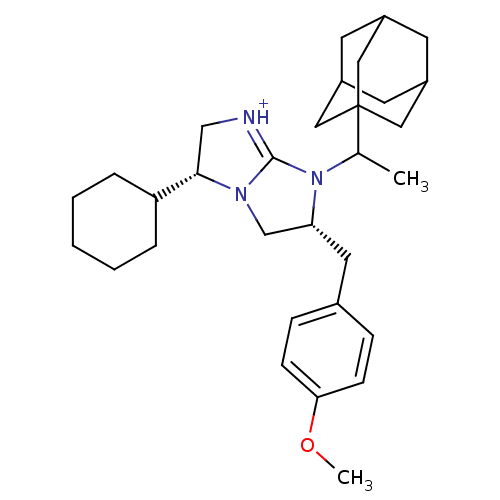
((3R,6R)-7-(1-Adamantan-1-yl-ethyl)-3-cyclohexyl-6-...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C[NH+]=C3N2C(C)C23CC4CC(CC(C4)C2)C3)C2CCCCC2)cc1 |r,c:12,TLB:15:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| Show InChI InChI=1S/C31H45N3O/c1-21(31-16-23-12-24(17-31)14-25(13-23)18-31)34-27(15-22-8-10-28(35-2)11-9-22)20-33-29(19-32-30(33)34)26-6-4-3-5-7-26/h8-11,21,23-27,29H,3-7,12-20H2,1-2H3/p+1/t21?,23?,24?,25?,27-,29+,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 341 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271919
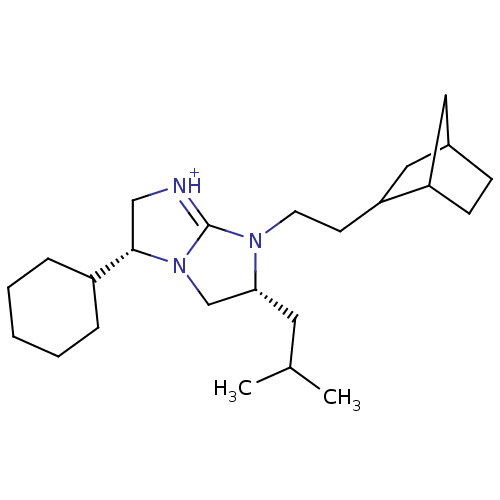
((3R,6R)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-3-cycl...)Show SMILES CC(C)C[C@@H]1CN2[C@@H](C[NH+]=C2N1CCC1CC2CCC1C2)C1CCCCC1 |r,c:9,THB:13:14:20:18.17| Show InChI InChI=1S/C24H41N3/c1-17(2)12-22-16-27-23(19-6-4-3-5-7-19)15-25-24(27)26(22)11-10-21-14-18-8-9-20(21)13-18/h17-23H,3-16H2,1-2H3/p+1/t18?,20?,21?,22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 359 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271957
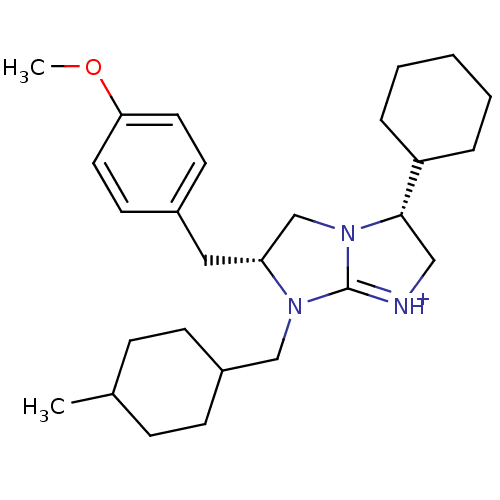
((3R,6R)-3-Cyclohexyl-6-(4-methoxy-benzyl)-7-(4-met...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C[NH+]=C3N2CC2CCC(C)CC2)C2CCCCC2)cc1 |r,wU:7.6,10.25,c:12,(5.32,1,;3.78,1.03,;2.98,-.28,;3.71,-1.64,;2.91,-2.95,;1.37,-2.9,;.57,-4.21,;-.97,-4.17,;-1.84,-2.9,;-3.32,-3.34,;-4.78,-2.82,;-5.72,-4.05,;-4.85,-5.32,;-3.36,-4.88,;-1.91,-5.4,;-1.47,-6.88,;.03,-7.24,;.46,-8.72,;1.95,-9.08,;3.02,-7.97,;4.51,-8.33,;2.58,-6.49,;1.08,-6.12,;-5.22,-1.34,;-4.16,-.23,;-4.59,1.24,;-6.09,1.6,;-7.15,.49,;-6.72,-1,;.63,-1.56,;1.43,-.25,)| Show InChI InChI=1S/C27H41N3O/c1-20-8-10-22(11-9-20)18-29-24(16-21-12-14-25(31-2)15-13-21)19-30-26(17-28-27(29)30)23-6-4-3-5-7-23/h12-15,20,22-24,26H,3-11,16-19H2,1-2H3/p+1/t20?,22?,24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 362 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369467

(CHEMBL435414)Show SMILES CC1CCC(CN2[C@H](CN3[C@H](CN=C23)C2CCCCC2)C2CCCCC2)CC1 |wD:7.22,10.15,t:12,(-.93,-15.5,;-.32,-14.08,;1.22,-13.92,;1.84,-12.51,;.94,-11.27,;1.56,-9.86,;.66,-8.62,;1.12,-7.16,;-.09,-6.26,;-1.34,-7.16,;-2.87,-7.16,;-3.36,-8.6,;-2.11,-9.51,;-.87,-8.62,;-3.71,-5.86,;-5.24,-5.95,;-6.06,-4.65,;-5.36,-3.28,;-3.83,-3.21,;-3,-4.51,;2.59,-6.69,;3.72,-7.74,;5.19,-7.27,;5.53,-5.76,;4.39,-4.72,;2.91,-5.19,;-.6,-11.43,;-1.22,-12.83,)| Show InChI InChI=1S/C25H43N3/c1-19-12-14-20(15-13-19)17-27-24(22-10-6-3-7-11-22)18-28-23(16-26-25(27)28)21-8-4-2-5-9-21/h19-24H,2-18H2,1H3/t19?,20?,23-,24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 365 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271958
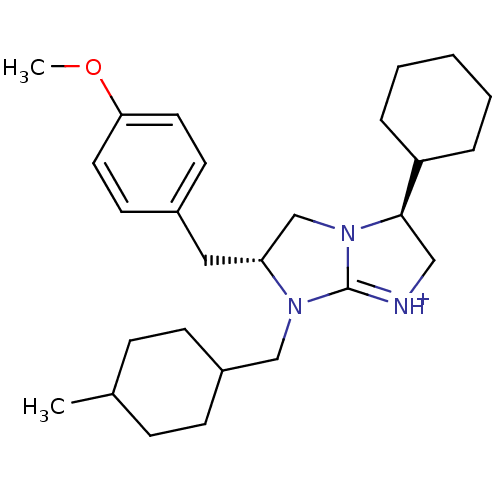
((3S,6R)-3-Cyclohexyl-6-(4-methoxy-benzyl)-7-(4-met...)Show SMILES COc1ccc(C[C@@H]2CN3[C@H](C[NH+]=C3N2CC2CCC(C)CC2)C2CCCCC2)cc1 |r,wU:7.6,wD:10.25,c:12,(39.97,1.29,;38.43,1.32,;37.63,.01,;38.36,-1.35,;37.56,-2.66,;36.02,-2.61,;35.22,-3.92,;33.68,-3.88,;32.81,-2.61,;31.33,-3.05,;29.87,-2.53,;28.93,-3.76,;29.8,-5.03,;31.29,-4.59,;32.74,-5.11,;33.18,-6.59,;32.12,-7.71,;30.63,-7.34,;29.57,-8.45,;30,-9.93,;28.94,-11.04,;31.5,-10.29,;32.57,-9.18,;29.43,-1.05,;30.49,.06,;30.06,1.53,;28.56,1.89,;27.5,.78,;27.93,-.71,;35.28,-1.27,;36.08,.04,)| Show InChI InChI=1S/C27H41N3O/c1-20-8-10-22(11-9-20)18-29-24(16-21-12-14-25(31-2)15-13-21)19-30-26(17-28-27(29)30)23-6-4-3-5-7-23/h12-15,20,22-24,26H,3-11,16-19H2,1-2H3/p+1/t20?,22?,24-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 369 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271959

((3S,6R)-6-(4-Methoxy-benzyl)-3-methyl-7-(4-methyl-...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C)C[NH+]=C3N2CC2CCC(C)CC2)cc1 |r,wU:7.6,wD:10.10,c:13,(2.71,-16.19,;1.17,-16.16,;.37,-17.47,;1.1,-18.83,;.3,-20.14,;-1.24,-20.09,;-2.04,-21.4,;-3.58,-21.36,;-4.45,-20.09,;-5.93,-20.53,;-7.39,-20.01,;-7.83,-18.53,;-8.33,-21.24,;-7.46,-22.51,;-5.97,-22.07,;-4.52,-22.59,;-4.08,-24.07,;-5.14,-25.19,;-6.63,-24.82,;-7.69,-25.93,;-7.26,-27.41,;-8.32,-28.52,;-5.76,-27.77,;-4.69,-26.66,;-1.98,-18.75,;-1.18,-17.44,)| Show InChI InChI=1S/C22H33N3O/c1-16-4-6-19(7-5-16)14-25-20(15-24-17(2)13-23-22(24)25)12-18-8-10-21(26-3)11-9-18/h8-11,16-17,19-20H,4-7,12-15H2,1-3H3/p+1/t16?,17-,19?,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 425 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271960
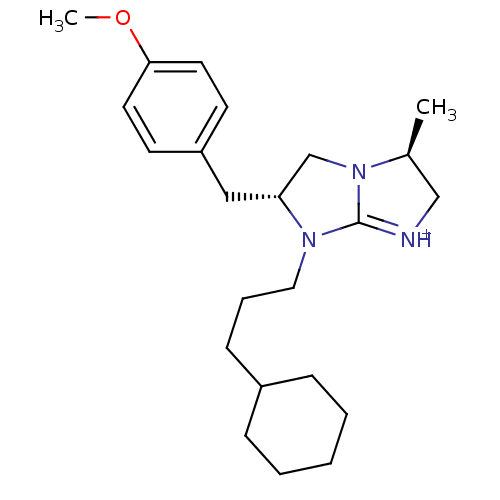
((3S,6R)-7-(3-Cyclohexyl-propyl)-6-(4-methoxy-benzy...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C)C[NH+]=C3N2CCCC2CCCCC2)cc1 |r,c:13| Show InChI InChI=1S/C23H35N3O/c1-18-16-24-23-25(14-6-9-19-7-4-3-5-8-19)21(17-26(18)23)15-20-10-12-22(27-2)13-11-20/h10-13,18-19,21H,3-9,14-17H2,1-2H3/p+1/t18-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 502 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271991
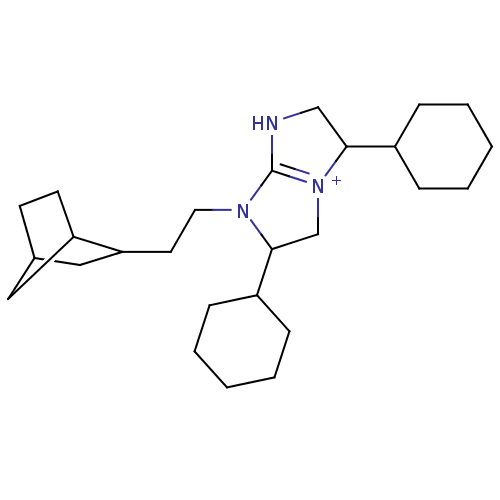
((2S,5R)-1-(2-{bicyclo[2.2.1]heptan-2-yl}ethyl)-2,5...)Show SMILES C(CN1C(C[N+]2=C1NCC2C1CCCCC1)C1CCCCC1)C1CC2CCC1C2 |c:5,THB:0:22:28:26.25| Show InChI InChI=1S/C26H43N3/c1-3-7-20(8-4-1)24-17-27-26-28(14-13-23-16-19-11-12-22(23)15-19)25(18-29(24)26)21-9-5-2-6-10-21/h19-25H,1-18H2/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 524 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271992
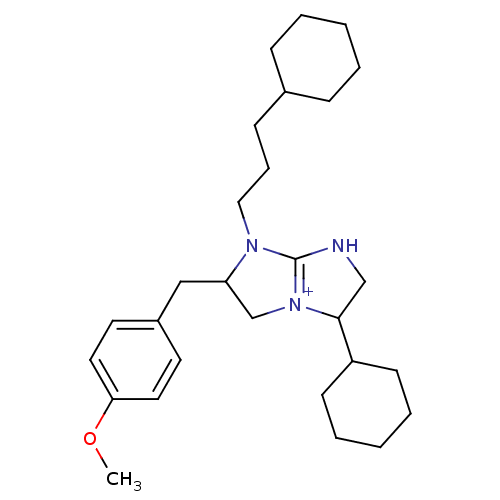
((2R,5R)-5-cyclohexyl-1-(3-cyclohexylpropyl)-2-[(4-...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CCCC2CCCCC2)cc1 |t:9| Show InChI InChI=1S/C28H43N3O/c1-32-26-16-14-23(15-17-26)19-25-21-31-27(24-12-6-3-7-13-24)20-29-28(31)30(25)18-8-11-22-9-4-2-5-10-22/h14-17,22,24-25,27H,2-13,18-21H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 547 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271991

((2S,5R)-1-(2-{bicyclo[2.2.1]heptan-2-yl}ethyl)-2,5...)Show SMILES C(CN1C(C[N+]2=C1NCC2C1CCCCC1)C1CCCCC1)C1CC2CCC1C2 |c:5,THB:0:22:28:26.25| Show InChI InChI=1S/C26H43N3/c1-3-7-20(8-4-1)24-17-27-26-28(14-13-23-16-19-11-12-22(23)15-19)25(18-29(24)26)21-9-5-2-6-10-21/h19-25H,1-18H2/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271994
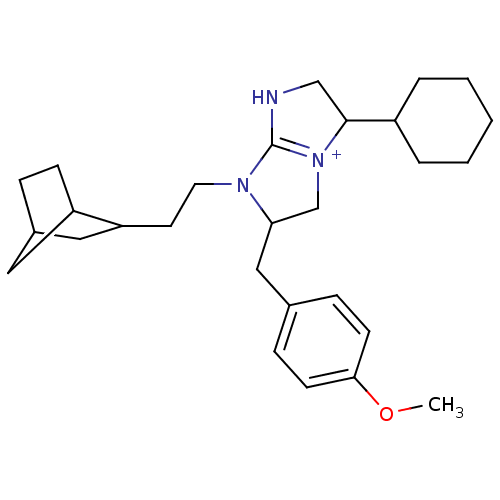
((2R,5S)-1-(2-{bicyclo[2.2.1]heptan-2-yl}ethyl)-5-c...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CCC2CC3CCC2C3)cc1 |t:9,THB:22:23:29:27.26| Show InChI InChI=1S/C28H41N3O/c1-32-26-11-8-20(9-12-26)17-25-19-31-27(22-5-3-2-4-6-22)18-29-28(31)30(25)14-13-24-16-21-7-10-23(24)15-21/h8-9,11-12,21-25,27H,2-7,10,13-19H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 715 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity towards Opioid receptor kappa 1 |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50272034
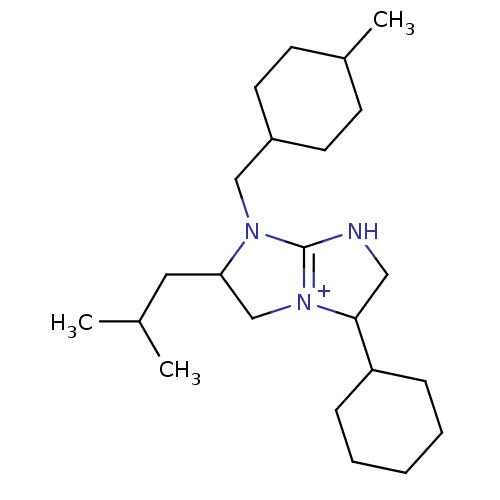
((2R,5R)-5-cyclohexyl-1-[(4-methylcyclohexyl)methyl...)Show SMILES CC(C)CC1C[N+]2=C(NCC2C2CCCCC2)N1CC1CCC(C)CC1 |t:6,(-.57,1.19,;.17,-.16,;1.71,-.2,;-.63,-1.47,;-2.17,-1.44,;-3.05,-.17,;-4.52,-.6,;-4.57,-2.15,;-6.05,-2.59,;-6.92,-1.32,;-5.98,-.08,;-6.42,1.39,;-5.36,2.5,;-5.79,3.98,;-7.29,4.34,;-8.36,3.22,;-7.92,1.74,;-3.11,-2.66,;-2.67,-4.14,;-3.73,-5.26,;-5.23,-4.9,;-6.28,-6.01,;-5.85,-7.49,;-6.91,-8.61,;-4.35,-7.85,;-3.29,-6.74,)| Show InChI InChI=1S/C23H41N3/c1-17(2)13-21-16-26-22(20-7-5-4-6-8-20)14-24-23(26)25(21)15-19-11-9-18(3)10-12-19/h17-22H,4-16H2,1-3H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 738 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50272035
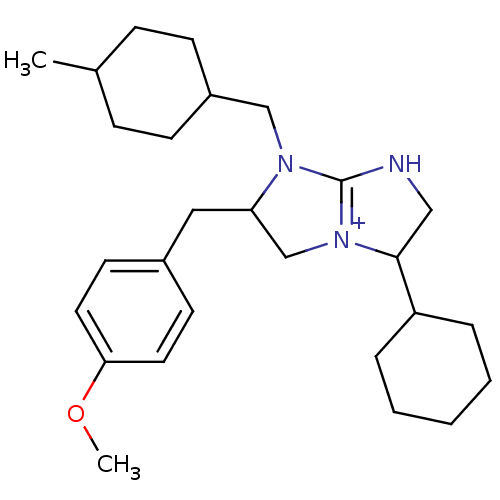
((2S,5R)-5-cyclohexyl-2-[(4-methoxyphenyl)methyl]-1...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CC2CCC(C)CC2)cc1 |t:9,(20.14,3.61,;18.6,3.65,;17.79,2.33,;18.53,.98,;17.73,-.34,;16.19,-.29,;15.39,-1.6,;13.85,-1.57,;12.97,-.3,;11.5,-.73,;11.45,-2.28,;9.98,-2.71,;9.1,-1.45,;10.04,-.21,;9.61,1.26,;10.66,2.37,;10.23,3.85,;8.73,4.21,;7.67,3.09,;8.1,1.61,;12.91,-2.79,;13.35,-4.27,;12.29,-5.39,;10.8,-5.03,;9.74,-6.14,;10.17,-7.62,;9.11,-8.74,;11.67,-7.98,;12.74,-6.87,;15.45,1.05,;16.25,2.36,)| Show InChI InChI=1S/C27H41N3O/c1-20-8-10-22(11-9-20)18-29-24(16-21-12-14-25(31-2)15-13-21)19-30-26(17-28-27(29)30)23-6-4-3-5-7-23/h12-15,20,22-24,26H,3-11,16-19H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 804 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50272036

((2R,5S)-1-(2-{bicyclo[2.2.1]heptan-2-yl}ethyl)-5-c...)Show SMILES CC(C)CC1C[N+]2=C(NCC2C2CCCCC2)N1CCC1CC2CCC1C2 |t:6,THB:19:20:26:24.23| Show InChI InChI=1S/C24H41N3/c1-17(2)12-22-16-27-23(19-6-4-3-5-7-19)15-25-24(27)26(22)11-10-21-14-18-8-9-20(21)13-18/h17-23H,3-16H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 827 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271994

((2R,5S)-1-(2-{bicyclo[2.2.1]heptan-2-yl}ethyl)-5-c...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CCC2CC3CCC2C3)cc1 |t:9,THB:22:23:29:27.26| Show InChI InChI=1S/C28H41N3O/c1-32-26-11-8-20(9-12-26)17-25-19-31-27(22-5-3-2-4-6-22)18-29-28(31)30(25)14-13-24-16-21-7-10-23(24)15-21/h8-9,11-12,21-25,27H,2-7,10,13-19H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 924 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50272038
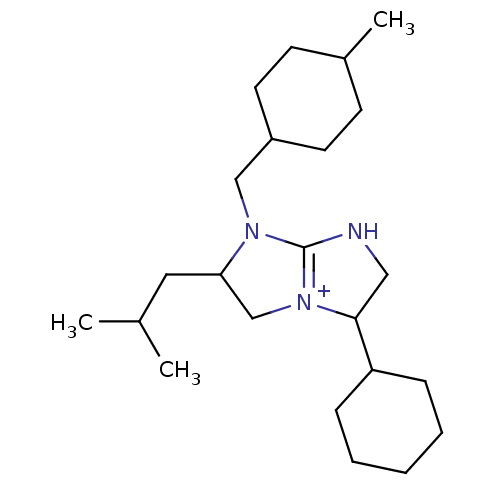
((2R,5S)-5-cyclohexyl-1-[(4-methylcyclohexyl)methyl...)Show SMILES CC(C)CC1C[N+]2=C(NCC2C2CCCCC2)N1CC1CCC(C)CC1 |t:6,(18.78,-19.11,;17.24,-19.08,;16.51,-17.72,;16.44,-20.39,;14.9,-20.35,;14.03,-19.08,;12.56,-19.52,;12.51,-21.07,;11.03,-21.5,;10.15,-20.23,;11.1,-19,;10.66,-17.53,;11.71,-16.42,;11.28,-14.94,;9.78,-14.58,;8.72,-15.69,;9.15,-17.17,;13.96,-21.58,;14.4,-23.06,;13.34,-24.18,;11.85,-23.81,;10.79,-24.92,;11.23,-26.4,;10.16,-27.51,;12.72,-26.76,;13.79,-25.65,)| Show InChI InChI=1S/C23H41N3/c1-17(2)13-21-16-26-22(20-7-5-4-6-8-20)14-24-23(26)25(21)15-19-11-9-18(3)10-12-19/h17-22H,4-16H2,1-3H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 999 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271708
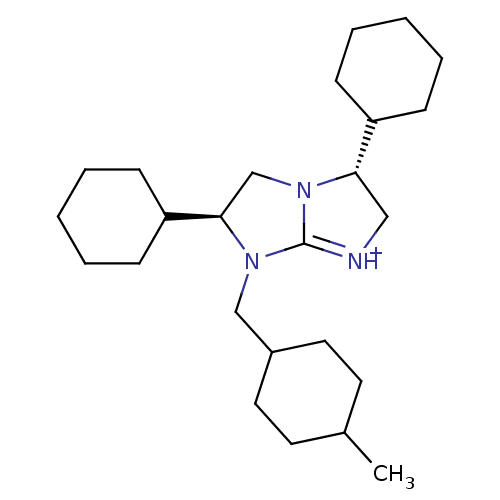
((2S,5R)-2,5-Dicyclohexyl-1-(4-methyl-cyclohexylmet...)Show SMILES CC1CCC(CN2[C@H](CN3[C@@H](C[NH+]=C23)C2CCCCC2)C2CCCCC2)CC1 |r,wU:10.15,wD:7.22,t:12,(10.65,-14.86,;11.71,-13.74,;11.28,-12.26,;12.34,-11.15,;13.83,-11.52,;14.89,-10.4,;14.46,-8.92,;15.39,-7.69,;14.52,-6.42,;13.05,-6.86,;11.59,-6.34,;10.65,-7.57,;11.52,-8.84,;13,-8.41,;11.15,-4.87,;12.21,-3.76,;11.77,-2.29,;10.28,-1.92,;9.21,-3.04,;9.65,-4.52,;16.93,-7.73,;17.67,-9.1,;19.2,-9.14,;20.01,-7.83,;19.28,-6.48,;17.73,-6.43,;14.28,-12.99,;13.21,-14.11,)| Show InChI InChI=1S/C25H43N3/c1-19-12-14-20(15-13-19)17-27-24(22-10-6-3-7-11-22)18-28-23(16-26-25(27)28)21-8-4-2-5-9-21/h19-24H,2-18H2,1H3/p+1/t19?,20?,23-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369462

(CHEMBL121933)Show SMILES COc1ccc(C[C@H]2CN3[C@H](CN=C3N2C(C)C23CC4CC(CC(C4)C2)C3)C2CCCCC2)cc1 |c:12,TLB:24:23:26:18.19.20,24:19:26:25.23.22,THB:22:23:18:26.21.20,22:21:18:25.23.24| Show InChI InChI=1S/C31H45N3O/c1-21(31-16-23-12-24(17-31)14-25(13-23)18-31)34-27(15-22-8-10-28(35-2)11-9-22)20-33-29(19-32-30(33)34)26-6-4-3-5-7-26/h8-11,21,23-27,29H,3-7,12-20H2,1-2H3/t21?,23?,24?,25?,27-,29+,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369459
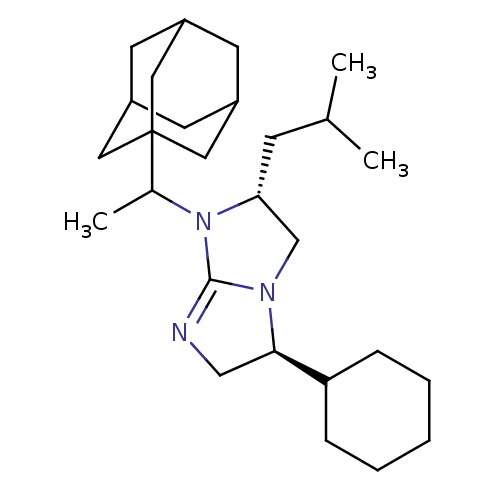
(CHEMBL127486)Show SMILES CC(C)C[C@@H]1CN2[C@H](CN=C2N1C(C)C12CC3CC(CC(C3)C1)C2)C1CCCCC1 |c:9,TLB:21:20:23:15.16.17,21:16:23:22.20.19,THB:19:20:15:23.18.17,19:18:15:22.20.21| Show InChI InChI=1S/C27H45N3/c1-18(2)9-24-17-29-25(23-7-5-4-6-8-23)16-28-26(29)30(24)19(3)27-13-20-10-21(14-27)12-22(11-20)15-27/h18-25H,4-17H2,1-3H3/t19?,20?,21?,22?,24-,25-,27?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271772

((3S,6S)-7-(3-Cyclohexyl-propyl)-6-(4-methoxy-benzy...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)C[NH+]=C3N2CCCC2CCCCC2)cc1 |r,c:13| Show InChI InChI=1S/C23H35N3O/c1-18-16-24-23-25(14-6-9-19-7-4-3-5-8-19)21(17-26(18)23)15-20-10-12-22(27-2)13-11-20/h10-13,18-19,21H,3-9,14-17H2,1-2H3/p+1/t18-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369455
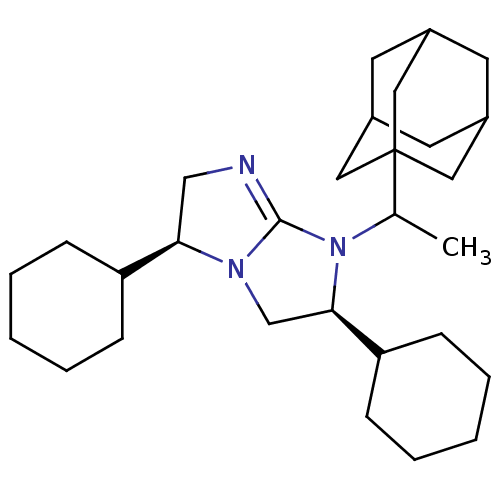
(CHEMBL127485)Show SMILES CC(N1[C@H](CN2[C@H](CN=C12)C1CCCCC1)C1CCCCC1)C12CC3CC(CC(C3)C1)C2 |t:8,TLB:29:28:31:23.24.25,29:24:31:30.28.27,THB:27:28:23:31.26.25,27:26:23:30.28.29| Show InChI InChI=1S/C29H47N3/c1-20(29-15-21-12-22(16-29)14-23(13-21)17-29)32-27(25-10-6-3-7-11-25)19-31-26(18-30-28(31)32)24-8-4-2-5-9-24/h20-27H,2-19H2,1H3/t20?,21?,22?,23?,26-,27-,29?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369453
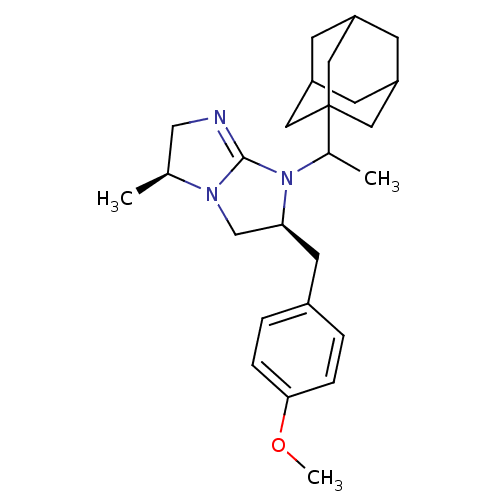
(CHEMBL127814)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)CN=C3N2C(C)C23CC4CC(CC(C4)C2)C3)cc1 |c:13,TLB:21:22:26:19.20.25,THB:21:20:26:27.22.23,23:22:19:26.24.25,23:24:19:27.22.21| Show InChI InChI=1S/C26H37N3O/c1-17-15-27-25-28(17)16-23(11-19-4-6-24(30-3)7-5-19)29(25)18(2)26-12-20-8-21(13-26)10-22(9-20)14-26/h4-7,17-18,20-23H,8-16H2,1-3H3/t17-,18?,20?,21?,22?,23-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271310
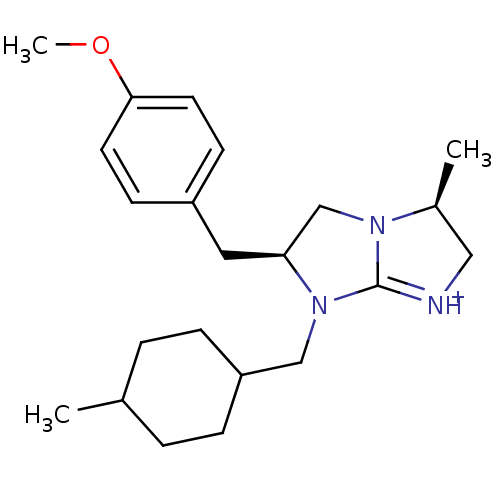
((3S,6S)-6-(4-Methoxy-benzyl)-3-methyl-7-(4-methyl-...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)C[NH+]=C3N2CC2CCC(C)CC2)cc1 |r,wD:10.10,7.6,c:13,(2.37,2.47,;.83,2.51,;.03,1.19,;.77,-.16,;-.03,-1.47,;-1.57,-1.43,;-2.37,-2.74,;-3.91,-2.7,;-4.79,-1.43,;-6.26,-1.87,;-7.72,-1.34,;-8.16,.12,;-8.66,-2.58,;-7.79,-3.85,;-6.31,-3.42,;-4.85,-3.93,;-4.41,-5.41,;-5.47,-6.52,;-6.97,-6.17,;-8.02,-7.28,;-7.59,-8.76,;-8.65,-9.87,;-6.09,-9.12,;-5.03,-8,;-2.31,-.09,;-1.51,1.23,)| Show InChI InChI=1S/C22H33N3O/c1-16-4-6-19(7-5-16)14-25-20(15-24-17(2)13-23-22(24)25)12-18-8-10-21(26-3)11-9-18/h8-11,16-17,19-20H,4-7,12-15H2,1-3H3/p+1/t16?,17-,19?,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271503
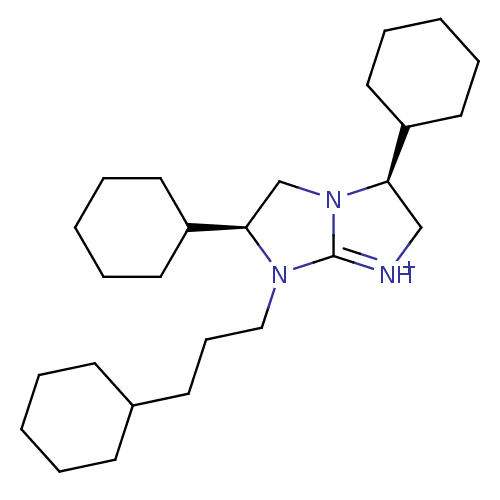
((3S,6S)-3,6-Dicyclohexyl-7-(3-cyclohexyl-propyl)-2...)Show SMILES C(CC1CCCCC1)CN1[C@H](CN2[C@H](C[NH+]=C12)C1CCCCC1)C1CCCCC1 |r,t:16| Show InChI InChI=1S/C26H45N3/c1-4-11-21(12-5-1)13-10-18-28-25(23-16-8-3-9-17-23)20-29-24(19-27-26(28)29)22-14-6-2-7-15-22/h21-25H,1-20H2/p+1/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369463

(CHEMBL125130)Show SMILES CC(C)C[C@@H]1CN2[C@@H](CN=C2N1C(C)C12CC3CC(CC(C3)C1)C2)C1CCCCC1 |c:9,TLB:21:20:23:15.16.17,21:16:23:22.20.19,THB:19:20:15:23.18.17,19:18:15:22.20.21| Show InChI InChI=1S/C27H45N3/c1-18(2)9-24-17-29-25(23-7-5-4-6-8-23)16-28-26(29)30(24)19(3)27-13-20-10-21(14-27)12-22(11-20)15-27/h18-25H,4-17H2,1-3H3/t19?,20?,21?,22?,24-,25+,27?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271453
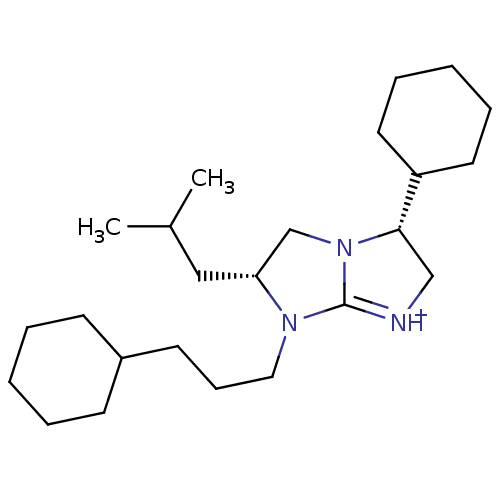
((3R,6R)-3-Cyclohexyl-7-(3-cyclohexyl-propyl)-6-iso...)Show SMILES CC(C)C[C@@H]1CN2[C@@H](C[NH+]=C2N1CCCC1CCCCC1)C1CCCCC1 |r,c:9| Show InChI InChI=1S/C24H43N3/c1-19(2)16-22-18-27-23(21-13-7-4-8-14-21)17-25-24(27)26(22)15-9-12-20-10-5-3-6-11-20/h19-23H,3-18H2,1-2H3/p+1/t22-,23+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271310

((3S,6S)-6-(4-Methoxy-benzyl)-3-methyl-7-(4-methyl-...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)C[NH+]=C3N2CC2CCC(C)CC2)cc1 |r,wD:10.10,7.6,c:13,(2.37,2.47,;.83,2.51,;.03,1.19,;.77,-.16,;-.03,-1.47,;-1.57,-1.43,;-2.37,-2.74,;-3.91,-2.7,;-4.79,-1.43,;-6.26,-1.87,;-7.72,-1.34,;-8.16,.12,;-8.66,-2.58,;-7.79,-3.85,;-6.31,-3.42,;-4.85,-3.93,;-4.41,-5.41,;-5.47,-6.52,;-6.97,-6.17,;-8.02,-7.28,;-7.59,-8.76,;-8.65,-9.87,;-6.09,-9.12,;-5.03,-8,;-2.31,-.09,;-1.51,1.23,)| Show InChI InChI=1S/C22H33N3O/c1-16-4-6-19(7-5-16)14-25-20(15-24-17(2)13-23-22(24)25)12-18-8-10-21(26-3)11-9-18/h8-11,16-17,19-20H,4-7,12-15H2,1-3H3/p+1/t16?,17-,19?,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369457

(CHEMBL122092)Show SMILES CC(N1[C@H](CN2[C@@H](C)CN=C12)C1CCCCC1)C12CC3CC(CC(C3)C1)C2 |t:9,TLB:20:21:25:18.19.24,THB:20:19:25:26.21.22,22:21:18:25.23.24,22:23:18:26.21.20| Show InChI InChI=1S/C24H39N3/c1-16-14-25-23-26(16)15-22(21-6-4-3-5-7-21)27(23)17(2)24-11-18-8-19(12-24)10-20(9-18)13-24/h16-22H,3-15H2,1-2H3/t16-,17?,18?,19?,20?,22+,24?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271869

((3S,6R)-7-(1-Adamantan-1-yl-ethyl)-6-(4-methoxy-be...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C)C[NH+]=C3N2C(C)C23CC4CC(CC(C4)C2)C3)cc1 |r,c:13,TLB:16:18:21.20.25:23,THB:19:20:23:27.18.26,19:18:21.20.25:23,26:18:21:25.24.23,26:24:21:27.19.18| Show InChI InChI=1S/C26H37N3O/c1-17-15-27-25-28(17)16-23(11-19-4-6-24(30-3)7-5-19)29(25)18(2)26-12-20-8-21(13-26)10-22(9-20)14-26/h4-7,17-18,20-23H,8-16H2,1-3H3/p+1/t17-,18?,20?,21?,22?,23+,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271455
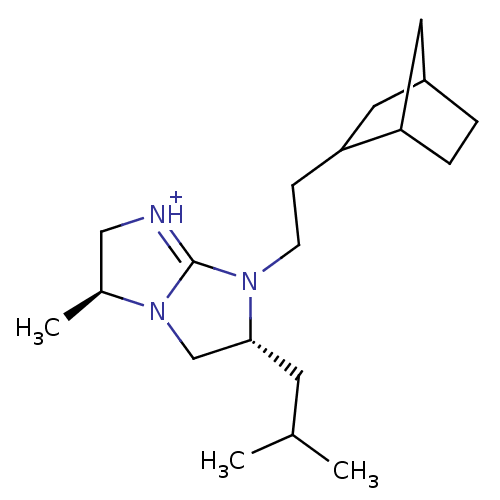
((3S,6R)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-6-isob...)Show SMILES CC(C)C[C@@H]1CN2[C@@H](C)C[NH+]=C2N1CCC1CC2CCC1C2 |r,c:10,THB:14:15:21:19.18| Show InChI InChI=1S/C19H33N3/c1-13(2)8-18-12-22-14(3)11-20-19(22)21(18)7-6-17-10-15-4-5-16(17)9-15/h13-18H,4-12H2,1-3H3/p+1/t14-,15?,16?,17?,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271958

((3S,6R)-3-Cyclohexyl-6-(4-methoxy-benzyl)-7-(4-met...)Show SMILES COc1ccc(C[C@@H]2CN3[C@H](C[NH+]=C3N2CC2CCC(C)CC2)C2CCCCC2)cc1 |r,wU:7.6,wD:10.25,c:12,(39.97,1.29,;38.43,1.32,;37.63,.01,;38.36,-1.35,;37.56,-2.66,;36.02,-2.61,;35.22,-3.92,;33.68,-3.88,;32.81,-2.61,;31.33,-3.05,;29.87,-2.53,;28.93,-3.76,;29.8,-5.03,;31.29,-4.59,;32.74,-5.11,;33.18,-6.59,;32.12,-7.71,;30.63,-7.34,;29.57,-8.45,;30,-9.93,;28.94,-11.04,;31.5,-10.29,;32.57,-9.18,;29.43,-1.05,;30.49,.06,;30.06,1.53,;28.56,1.89,;27.5,.78,;27.93,-.71,;35.28,-1.27,;36.08,.04,)| Show InChI InChI=1S/C27H41N3O/c1-20-8-10-22(11-9-20)18-29-24(16-21-12-14-25(31-2)15-13-21)19-30-26(17-28-27(29)30)23-6-4-3-5-7-23/h12-15,20,22-24,26H,3-11,16-19H2,1-2H3/p+1/t20?,22?,24-,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271456
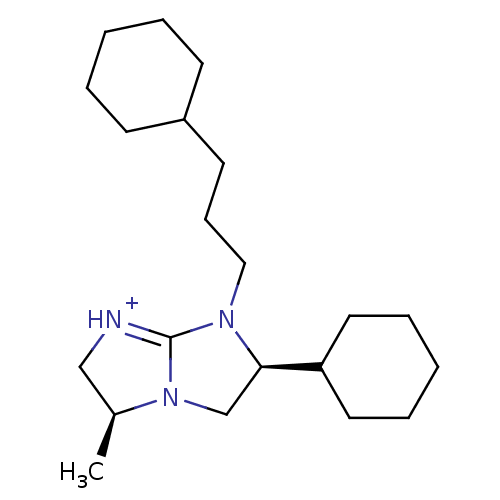
((3S,6S)-6-Cyclohexyl-7-(3-cyclohexyl-propyl)-3-met...)Show SMILES C[C@H]1C[NH+]=C2N(CCCC3CCCCC3)[C@H](CN12)C1CCCCC1 |r,t:3| Show InChI InChI=1S/C21H37N3/c1-17-15-22-21-23(14-8-11-18-9-4-2-5-10-18)20(16-24(17)21)19-12-6-3-7-13-19/h17-20H,2-16H2,1H3/p+1/t17-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271992

((2R,5R)-5-cyclohexyl-1-(3-cyclohexylpropyl)-2-[(4-...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CCCC2CCCCC2)cc1 |t:9| Show InChI InChI=1S/C28H43N3O/c1-32-26-16-14-23(15-17-26)19-25-21-31-27(24-12-6-3-7-13-24)20-29-28(31)30(25)18-8-11-22-9-4-2-5-10-22/h14-17,22,24-25,27H,2-13,18-21H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50272035

((2S,5R)-5-cyclohexyl-2-[(4-methoxyphenyl)methyl]-1...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CC2CCC(C)CC2)cc1 |t:9,(20.14,3.61,;18.6,3.65,;17.79,2.33,;18.53,.98,;17.73,-.34,;16.19,-.29,;15.39,-1.6,;13.85,-1.57,;12.97,-.3,;11.5,-.73,;11.45,-2.28,;9.98,-2.71,;9.1,-1.45,;10.04,-.21,;9.61,1.26,;10.66,2.37,;10.23,3.85,;8.73,4.21,;7.67,3.09,;8.1,1.61,;12.91,-2.79,;13.35,-4.27,;12.29,-5.39,;10.8,-5.03,;9.74,-6.14,;10.17,-7.62,;9.11,-8.74,;11.67,-7.98,;12.74,-6.87,;15.45,1.05,;16.25,2.36,)| Show InChI InChI=1S/C27H41N3O/c1-20-8-10-22(11-9-20)18-29-24(16-21-12-14-25(31-2)15-13-21)19-30-26(17-28-27(29)30)23-6-4-3-5-7-23/h12-15,20,22-24,26H,3-11,16-19H2,1-2H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271816

((3S,6S)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-6-(4-m...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)C[NH+]=C3N2CCC2CC3CCC2C3)cc1 |r,c:13,THB:17:18:24:22.21| Show InChI InChI=1S/C23H33N3O/c1-16-14-24-23-25(10-9-20-12-18-3-6-19(20)11-18)21(15-26(16)23)13-17-4-7-22(27-2)8-5-17/h4-5,7-8,16,18-21H,3,6,9-15H2,1-2H3/p+1/t16-,18?,19?,20?,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271994

((2R,5S)-1-(2-{bicyclo[2.2.1]heptan-2-yl}ethyl)-5-c...)Show SMILES COc1ccc(CC2C[N+]3=C(NCC3C3CCCCC3)N2CCC2CC3CCC2C3)cc1 |t:9,THB:22:23:29:27.26| Show InChI InChI=1S/C28H41N3O/c1-32-26-11-8-20(9-12-26)17-25-19-31-27(22-5-3-2-4-6-22)18-29-28(31)30(25)14-13-24-16-21-7-10-23(24)15-21/h8-9,11-12,21-25,27H,2-7,10,13-19H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271960

((3S,6R)-7-(3-Cyclohexyl-propyl)-6-(4-methoxy-benzy...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C)C[NH+]=C3N2CCCC2CCCCC2)cc1 |r,c:13| Show InChI InChI=1S/C23H35N3O/c1-18-16-24-23-25(14-6-9-19-7-4-3-5-8-19)21(17-26(18)23)15-20-10-12-22(27-2)13-11-20/h10-13,18-19,21H,3-9,14-17H2,1-2H3/p+1/t18-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271917

((3R,6R)-7-(1-Adamantan-1-yl-ethyl)-3-cyclohexyl-6-...)Show SMILES COc1ccc(C[C@@H]2CN3[C@@H](C[NH+]=C3N2C(C)C23CC4CC(CC(C4)C2)C3)C2CCCCC2)cc1 |r,c:12,TLB:15:17:20.19.24:22,THB:18:19:22:26.17.25,18:17:20.19.24:22,25:17:20:24.23.22,25:23:20:26.18.17| Show InChI InChI=1S/C31H45N3O/c1-21(31-16-23-12-24(17-31)14-25(13-23)18-31)34-27(15-22-8-10-28(35-2)11-9-22)20-33-29(19-32-30(33)34)26-6-4-3-5-7-26/h8-11,21,23-27,29H,3-7,12-20H2,1-2H3/p+1/t21?,23?,24?,25?,27-,29+,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271457
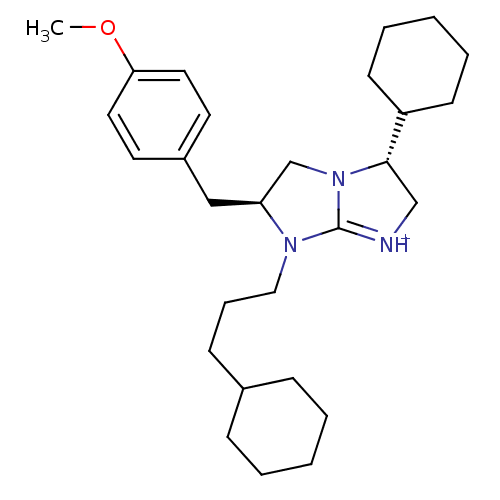
((2S,5R)-5-cyclohexyl-1-(3-cyclohexylpropyl)-2-[(4-...)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C[NH+]=C3N2CCCC2CCCCC2)C2CCCCC2)cc1 |r,c:12| Show InChI InChI=1S/C28H43N3O/c1-32-26-16-14-23(15-17-26)19-25-21-31-27(24-12-6-3-7-13-24)20-29-28(31)30(25)18-8-11-22-9-4-2-5-10-22/h14-17,22,24-25,27H,2-13,18-21H2,1H3/p+1/t25-,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369466
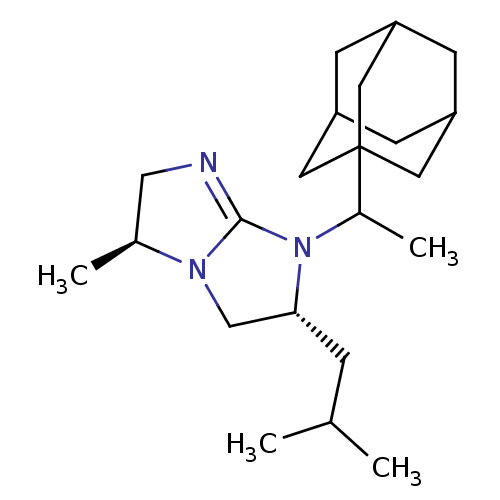
(CHEMBL124028)Show SMILES CC(C)C[C@@H]1CN2[C@@H](C)CN=C2N1C(C)C12CC3CC(CC(C3)C1)C2 |c:10,TLB:18:19:23:16.17.22,THB:18:17:23:24.19.20,20:19:16:23.21.22,20:21:16:24.19.18| Show InChI InChI=1S/C22H37N3/c1-14(2)5-20-13-24-15(3)12-23-21(24)25(20)16(4)22-9-17-6-18(10-22)8-19(7-17)11-22/h14-20H,5-13H2,1-4H3/t15-,16?,17?,18?,19?,20+,22?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50272038

((2R,5S)-5-cyclohexyl-1-[(4-methylcyclohexyl)methyl...)Show SMILES CC(C)CC1C[N+]2=C(NCC2C2CCCCC2)N1CC1CCC(C)CC1 |t:6,(18.78,-19.11,;17.24,-19.08,;16.51,-17.72,;16.44,-20.39,;14.9,-20.35,;14.03,-19.08,;12.56,-19.52,;12.51,-21.07,;11.03,-21.5,;10.15,-20.23,;11.1,-19,;10.66,-17.53,;11.71,-16.42,;11.28,-14.94,;9.78,-14.58,;8.72,-15.69,;9.15,-17.17,;13.96,-21.58,;14.4,-23.06,;13.34,-24.18,;11.85,-23.81,;10.79,-24.92,;11.23,-26.4,;10.16,-27.51,;12.72,-26.76,;13.79,-25.65,)| Show InChI InChI=1S/C23H41N3/c1-17(2)13-21-16-26-22(20-7-5-4-6-8-20)14-24-23(26)25(21)15-19-11-9-18(3)10-12-19/h17-22H,4-16H2,1-3H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271500
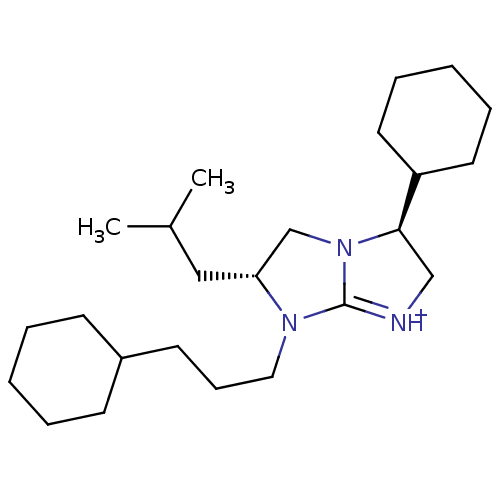
((3S,6R)-3-Cyclohexyl-7-(3-cyclohexyl-propyl)-6-iso...)Show SMILES CC(C)C[C@@H]1CN2[C@H](C[NH+]=C2N1CCCC1CCCCC1)C1CCCCC1 |r,c:9| Show InChI InChI=1S/C24H43N3/c1-19(2)16-22-18-27-23(21-13-7-4-8-14-21)17-25-24(27)26(22)15-9-12-20-10-5-3-6-11-20/h19-23H,3-18H2,1-2H3/p+1/t22-,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369453

(CHEMBL127814)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](C)CN=C3N2C(C)C23CC4CC(CC(C4)C2)C3)cc1 |c:13,TLB:21:22:26:19.20.25,THB:21:20:26:27.22.23,23:22:19:26.24.25,23:24:19:27.22.21| Show InChI InChI=1S/C26H37N3O/c1-17-15-27-25-28(17)16-23(11-19-4-6-24(30-3)7-5-19)29(25)18(2)26-12-20-8-21(13-26)10-22(9-20)14-26/h4-7,17-18,20-23H,8-16H2,1-3H3/t17-,18?,20?,21?,22?,23-,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibition of Opioid receptor kappa 1 binding |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271501
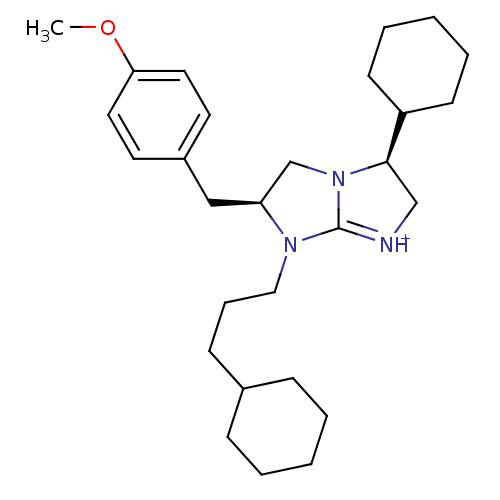
((3S,6S)-3-Cyclohexyl-7-(3-cyclohexyl-propyl)-6-(4-...)Show SMILES COc1ccc(C[C@H]2CN3[C@H](C[NH+]=C3N2CCCC2CCCCC2)C2CCCCC2)cc1 |r,c:12| Show InChI InChI=1S/C28H43N3O/c1-32-26-16-14-23(15-17-26)19-25-21-31-27(24-12-6-3-7-13-24)20-29-28(31)30(25)18-8-11-22-9-4-2-5-10-22/h14-17,22,24-25,27H,2-13,18-21H2,1H3/p+1/t25-,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271502
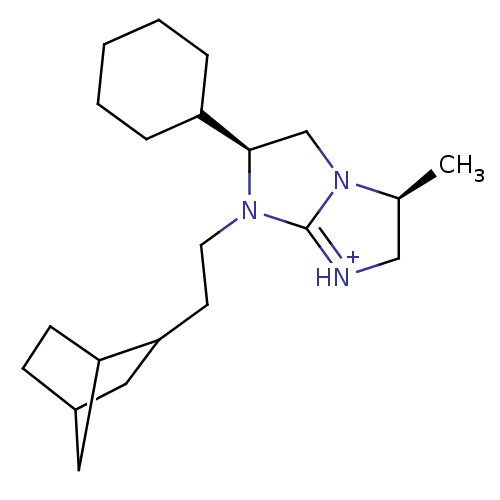
((3S,6S)-7-(2-Bicyclo[2.2.1]hept-2-yl-ethyl)-6-cycl...)Show SMILES C[C@H]1C[NH+]=C2N(CCC3CC4CCC3C4)[C@H](CN12)C1CCCCC1 |r,t:3,THB:7:8:14:12.11| Show InChI InChI=1S/C21H35N3/c1-15-13-22-21-23(10-9-19-12-16-7-8-18(19)11-16)20(14-24(15)21)17-5-3-2-4-6-17/h15-20H,2-14H2,1H3/p+1/t15-,16?,18?,19?,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271503

((3S,6S)-3,6-Dicyclohexyl-7-(3-cyclohexyl-propyl)-2...)Show SMILES C(CC1CCCCC1)CN1[C@H](CN2[C@H](C[NH+]=C12)C1CCCCC1)C1CCCCC1 |r,t:16| Show InChI InChI=1S/C26H45N3/c1-4-11-21(12-5-1)13-10-18-28-25(23-16-8-3-9-17-23)20-29-24(19-27-26(28)29)22-14-6-2-7-15-22/h21-25H,1-20H2/p+1/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369462

(CHEMBL121933)Show SMILES COc1ccc(C[C@H]2CN3[C@H](CN=C3N2C(C)C23CC4CC(CC(C4)C2)C3)C2CCCCC2)cc1 |c:12,TLB:24:23:26:18.19.20,24:19:26:25.23.22,THB:22:23:18:26.21.20,22:21:18:25.23.24| Show InChI InChI=1S/C31H45N3O/c1-21(31-16-23-12-24(17-31)14-25(13-23)18-31)34-27(15-22-8-10-28(35-2)11-9-22)20-33-29(19-32-30(33)34)26-6-4-3-5-7-26/h8-11,21,23-27,29H,3-7,12-20H2,1-2H3/t21?,23?,24?,25?,27-,29+,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271815
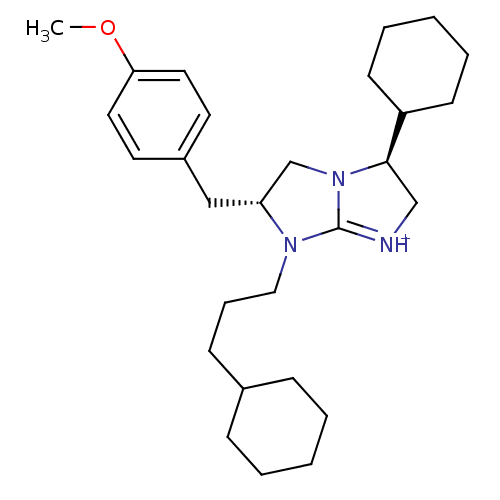
((3S,6R)-3-Cyclohexyl-7-(3-cyclohexyl-propyl)-6-(4-...)Show SMILES COc1ccc(C[C@@H]2CN3[C@H](C[NH+]=C3N2CCCC2CCCCC2)C2CCCCC2)cc1 |r,c:12| Show InChI InChI=1S/C28H43N3O/c1-32-26-16-14-23(15-17-26)19-25-21-31-27(24-12-6-3-7-13-24)20-29-28(31)30(25)18-8-11-22-9-4-2-5-10-22/h14-17,22,24-25,27H,2-13,18-21H2,1H3/p+1/t25-,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271817

((3S,6S)-6-Cyclohexyl-3-methyl-7-(4-methyl-cyclohex...)Show SMILES C[C@H]1C[NH+]=C2N(CC3CCC(C)CC3)[C@H](CN12)C1CCCCC1 |r,wD:14.19,1.0,t:3,(26.73,-19.75,;27.16,-21.22,;26.23,-22.46,;27.08,-23.72,;28.57,-23.29,;30.02,-23.8,;30.46,-25.27,;29.4,-26.39,;27.91,-26.03,;26.85,-27.14,;27.29,-28.62,;26.23,-29.73,;28.79,-28.98,;29.86,-27.86,;30.96,-22.57,;30.09,-21.31,;28.62,-21.74,;32.5,-22.61,;33.23,-23.96,;34.76,-24,;35.57,-22.69,;34.84,-21.33,;33.29,-21.29,)| Show InChI InChI=1S/C20H35N3/c1-15-8-10-17(11-9-15)13-23-19(18-6-4-3-5-7-18)14-22-16(2)12-21-20(22)23/h15-19H,3-14H2,1-2H3/p+1/t15?,16-,17?,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271819
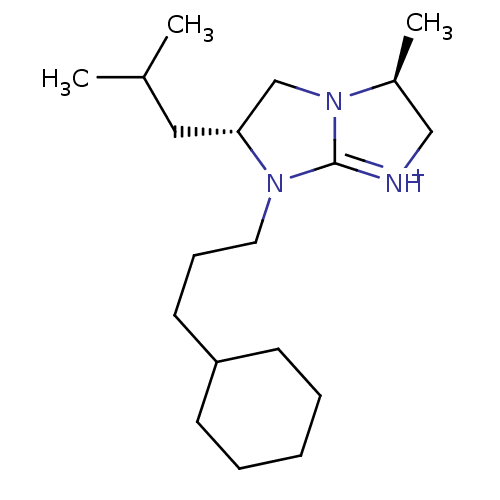
((3S,6R)-7-(3-Cyclohexyl-propyl)-6-isobutyl-3-methy...)Show SMILES CC(C)C[C@@H]1CN2[C@@H](C)C[NH+]=C2N1CCCC1CCCCC1 |r,c:10| Show InChI InChI=1S/C19H35N3/c1-15(2)12-18-14-22-16(3)13-20-19(22)21(18)11-7-10-17-8-5-4-6-9-17/h15-18H,4-14H2,1-3H3/p+1/t16-,18+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271870

((3S,6R)-6-Isobutyl-3-methyl-7-(4-methyl-cyclohexyl...)Show SMILES CC(C)C[C@@H]1CN2[C@@H](C)C[NH+]=C2N1CC1CCC(C)CC1 |r,wU:4.3,wD:7.7,c:10,(14.63,-41.05,;13.09,-41.01,;12.35,-39.66,;12.29,-42.33,;10.74,-42.29,;9.86,-41.02,;8.39,-41.45,;6.92,-40.93,;6.49,-39.45,;5.98,-42.18,;6.85,-43.44,;8.34,-43.01,;9.8,-43.52,;10.24,-45,;9.18,-46.12,;7.69,-45.76,;6.63,-46.87,;7.07,-48.35,;6.01,-49.46,;8.57,-48.71,;9.63,-47.59,)| Show InChI InChI=1S/C18H33N3/c1-13(2)9-17-12-20-15(4)10-19-18(20)21(17)11-16-7-5-14(3)6-8-16/h13-17H,5-12H2,1-4H3/p+1/t14?,15-,16?,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369465

(CHEMBL125660)Show SMILES CC(N1[C@H](CN2[C@@H](CN=C12)C1CCCCC1)C1CCCCC1)C12CC3CC(CC(C3)C1)C2 |t:8,TLB:29:28:31:23.24.25,29:24:31:30.28.27,THB:27:28:23:31.26.25,27:26:23:30.28.29| Show InChI InChI=1S/C29H47N3/c1-20(29-15-21-12-22(16-29)14-23(13-21)17-29)32-27(25-10-6-3-7-11-25)19-31-26(18-30-28(31)32)24-8-4-2-5-9-24/h20-27H,2-19H2,1H3/t20?,21?,22?,23?,26-,27+,29?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369464

(CHEMBL124207)Show SMILES COc1ccc(C[C@H]2CN3[C@@H](CN=C3N2C(C)C23CC4CC(CC(C4)C2)C3)C2CCCCC2)cc1 |c:12,TLB:24:23:26:18.19.20,24:19:26:25.23.22,THB:22:23:18:26.21.20,22:21:18:25.23.24| Show InChI InChI=1S/C31H45N3O/c1-21(31-16-23-12-24(17-31)14-25(13-23)18-31)34-27(15-22-8-10-28(35-2)11-9-22)20-33-29(19-32-30(33)34)26-6-4-3-5-7-26/h8-11,21,23-27,29H,3-7,12-20H2,1-2H3/t21?,23?,24?,25?,27-,29-,31?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50271540
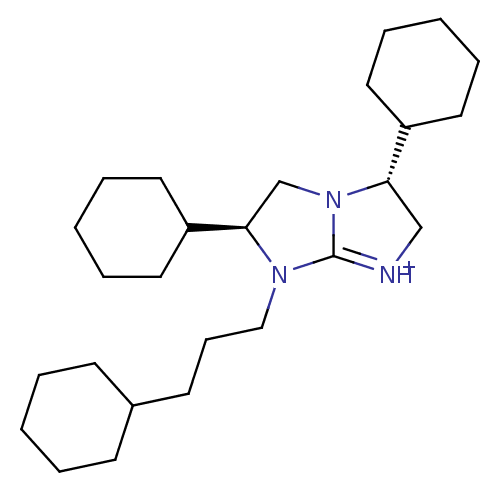
((3R,6S)-3,6-Dicyclohexyl-7-(3-cyclohexyl-propyl)-2...)Show SMILES C(CC1CCCCC1)CN1[C@H](CN2[C@@H](C[NH+]=C12)C1CCCCC1)C1CCCCC1 |r,t:16| Show InChI InChI=1S/C26H45N3/c1-4-11-21(12-5-1)13-10-18-28-25(23-16-8-3-9-17-23)20-29-24(19-27-26(28)29)22-14-6-2-7-15-22/h21-25H,1-20H2/p+1/t24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50369458
(CHEMBL433845)Show SMILES COc1ccc(C[C@@H]2CN3[C@H](CN=C3N2C(C)C23CC4CC(CC(C4)C2)C3)C2CCCCC2)cc1 |c:12,TLB:24:23:26:18.19.20,24:19:26:25.23.22,THB:22:23:18:26.21.20,22:21:18:25.23.24| Show InChI InChI=1S/C31H45N3O/c1-21(31-16-23-12-24(17-31)14-25(13-23)18-31)34-27(15-22-8-10-28(35-2)11-9-22)20-33-29(19-32-30(33)34)26-6-4-3-5-7-26/h8-11,21,23-27,29H,3-7,12-20H2,1-2H3/t21?,23?,24?,25?,27-,29-,31?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Inhibitory activity for kappa opioid receptor |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50369454

(CARBAMIC ACID) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PubMed
| n/a | n/a | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Enzyme inhibitory activity towards Acetylcholinesterase |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50369461

(THIAZOLIDINONE)Show InChI InChI=1S/C3H5NOS/c5-3-1-4-2-6-3/h4H,1-2H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Torrey Pines Institute for Molecular Studies
Curated by ChEMBL
| Assay Description
Enzyme inhibitory activity towards Prostaglandin G/H synthase 1 |
J Med Chem 42: 3743-78 (1999)
BindingDB Entry DOI: 10.7270/Q22Z167W |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data