

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50422300 (CHEMBL2364563) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Compounds was tested for its ability to stimulate phospholipase C activity at the P2Y purinoceptor 1 in turkey erythrocyte membranes. | J Med Chem 42: 5325-37 (2000) BindingDB Entry DOI: 10.7270/Q2V988S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50422302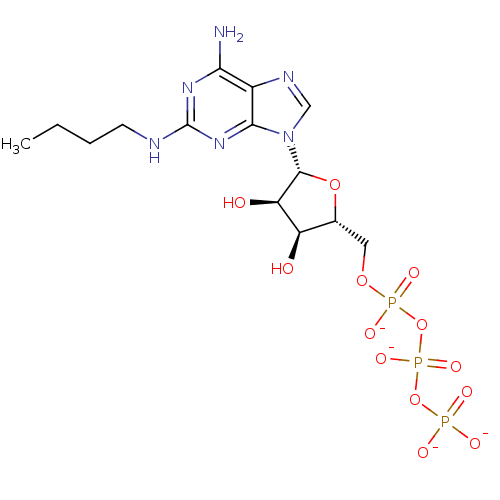 (CHEMBL2364568) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 39 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Compounds was tested for its ability to stimulate phospholipase C activity at the P2Y purinoceptor 1 in turkey erythrocyte membranes. | J Med Chem 42: 5325-37 (2000) BindingDB Entry DOI: 10.7270/Q2V988S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50422304 (CHEMBL2364567) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Compounds was tested for its ability to stimulate phospholipase C activity at the P2Y purinoceptor 1 in turkey erythrocyte membranes. | J Med Chem 42: 5325-37 (2000) BindingDB Entry DOI: 10.7270/Q2V988S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50422299 (CHEMBL2364566) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.71E+3 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Compounds was tested for its ability to stimulate phospholipase C activity at the P2Y purinoceptor 1 in turkey erythrocyte membranes. | J Med Chem 42: 5325-37 (2000) BindingDB Entry DOI: 10.7270/Q2V988S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50422301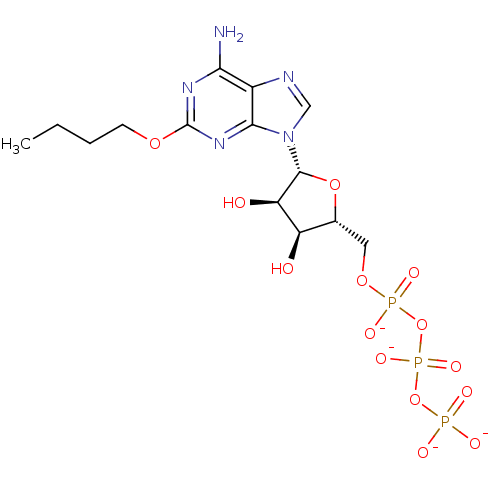 (CHEMBL2364565) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 88 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Compounds was tested for its ability to stimulate phospholipase C activity at the P2Y purinoceptor 1 in turkey erythrocyte membranes. | J Med Chem 42: 5325-37 (2000) BindingDB Entry DOI: 10.7270/Q2V988S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Meleagris gallopavo) | BDBM50422303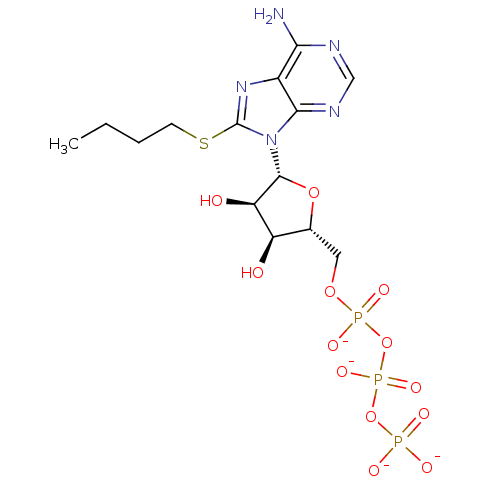 (CHEMBL2364564) | MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Compounds was tested for its ability to stimulate phospholipase C activity at the P2Y purinoceptor 1 in turkey erythrocyte membranes. | J Med Chem 42: 5325-37 (2000) BindingDB Entry DOI: 10.7270/Q2V988S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||