 Found 41 hits of Enzyme Inhibition Constant Data
Found 41 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085629
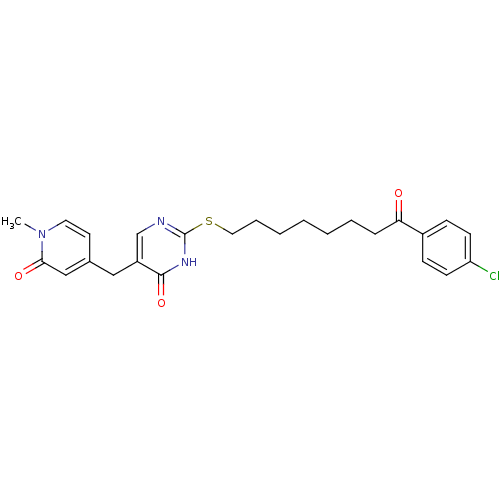
(2-[8-(4-Chloro-phenyl)-8-oxo-octylsulfanyl]-5-(1-m...)Show SMILES Cn1ccc(Cc2cnc(SCCCCCCCC(=O)c3ccc(Cl)cc3)[nH]c2=O)cc1=O Show InChI InChI=1S/C25H28ClN3O3S/c1-29-13-12-18(16-23(29)31)15-20-17-27-25(28-24(20)32)33-14-6-4-2-3-5-7-22(30)19-8-10-21(26)11-9-19/h8-13,16-17H,2-7,14-15H2,1H3,(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
The compound was tested for inhibition of Lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085626

(2-[8-(4-Chloro-phenyl)-8-oxo-octylsulfanyl]-5-pyri...)Show SMILES Clc1ccc(cc1)C(=O)CCCCCCCSc1ncc(Cc2cncnc2)c(=O)[nH]1 Show InChI InChI=1S/C23H25ClN4O2S/c24-20-9-7-18(8-10-20)21(29)6-4-2-1-3-5-11-31-23-27-15-19(22(30)28-23)12-17-13-25-16-26-14-17/h7-10,13-16H,1-6,11-12H2,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085655

(2-[8-(4-Chloro-phenyl)-8-oxo-octyloxy]-5-pyridin-3...)Show SMILES Clc1ccc(cc1)C(=O)CCCCCCCOc1ncc(Cc2cccnc2)c(=O)[nH]1 Show InChI InChI=1S/C24H26ClN3O3/c25-21-11-9-19(10-12-21)22(29)8-4-2-1-3-5-14-31-24-27-17-20(23(30)28-24)15-18-7-6-13-26-16-18/h6-7,9-13,16-17H,1-5,8,14-15H2,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085629

(2-[8-(4-Chloro-phenyl)-8-oxo-octylsulfanyl]-5-(1-m...)Show SMILES Cn1ccc(Cc2cnc(SCCCCCCCC(=O)c3ccc(Cl)cc3)[nH]c2=O)cc1=O Show InChI InChI=1S/C25H28ClN3O3S/c1-29-13-12-18(16-23(29)31)15-20-17-27-25(28-24(20)32)33-14-6-4-2-3-5-7-22(30)19-8-10-21(26)11-9-19/h8-13,16-17H,2-7,14-15H2,1H3,(H,27,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085616

(2-[8-(4-Chloro-phenyl)-8-oxo-octylsulfanyl]-5-pyri...)Show SMILES Clc1ccc(cc1)C(=O)CCCCCCCSc1ncc(Cc2cccnc2)c(=O)[nH]1 Show InChI InChI=1S/C24H26ClN3O2S/c25-21-11-9-19(10-12-21)22(29)8-4-2-1-3-5-14-31-24-27-17-20(23(30)28-24)15-18-7-6-13-26-16-18/h6-7,9-13,16-17H,1-5,8,14-15H2,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085634

(2-[8-(4-Chloro-phenyl)-octylsulfanyl]-5-pyridin-3-...)Show SMILES Clc1ccc(CCCCCCCCSc2ncc(Cc3cccnc3)c(=O)[nH]2)cc1 Show InChI InChI=1S/C24H28ClN3OS/c25-22-12-10-19(11-13-22)8-5-3-1-2-4-6-15-30-24-27-18-21(23(29)28-24)16-20-9-7-14-26-17-20/h7,9-14,17-18H,1-6,8,15-16H2,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085620

(2-[7-(4-Chloro-phenoxy)-heptylsulfanyl]-5-pyridin-...)Show SMILES Clc1ccc(OCCCCCCCSc2ncc(Cc3cccnc3)c(=O)[nH]2)cc1 Show InChI InChI=1S/C23H26ClN3O2S/c24-20-8-10-21(11-9-20)29-13-4-2-1-3-5-14-30-23-26-17-19(22(28)27-23)15-18-7-6-12-25-16-18/h6-12,16-17H,1-5,13-15H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085628

(2-[7-(4-Chloro-phenylsulfanyl)-heptylsulfanyl]-5-p...)Show SMILES Clc1ccc(SCCCCCCCSc2ncc(Cc3cccnc3)c(=O)[nH]2)cc1 Show InChI InChI=1S/C23H26ClN3OS2/c24-20-8-10-21(11-9-20)29-13-4-2-1-3-5-14-30-23-26-17-19(22(28)27-23)15-18-7-6-12-25-16-18/h6-12,16-17H,1-5,13-15H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085622

(5-(1-Methyl-2-oxo-1,2-dihydro-pyridin-4-ylmethyl)-...)Show SMILES Cn1ccc(Cc2cnc(SCCCCCCCCc3ccccc3)[nH]c2=O)cc1=O Show InChI InChI=1S/C25H31N3O2S/c1-28-15-14-21(18-23(28)29)17-22-19-26-25(27-24(22)30)31-16-10-5-3-2-4-7-11-20-12-8-6-9-13-20/h6,8-9,12-15,18-19H,2-5,7,10-11,16-17H2,1H3,(H,26,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085625
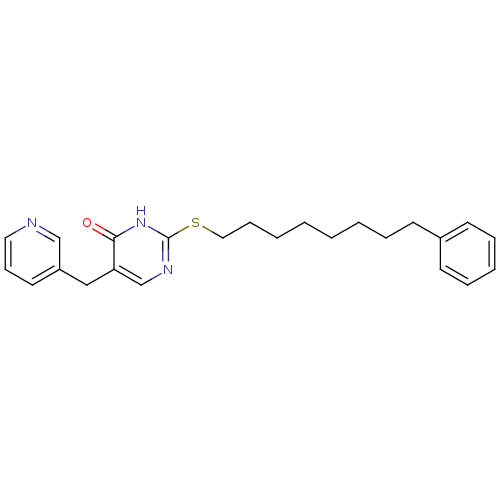
(2-(8-Phenyl-octylsulfanyl)-5-pyridin-3-ylmethyl-1H...)Show InChI InChI=1S/C24H29N3OS/c28-23-22(17-21-14-10-15-25-18-21)19-26-24(27-23)29-16-9-4-2-1-3-6-11-20-12-7-5-8-13-20/h5,7-8,10,12-15,18-19H,1-4,6,9,11,16-17H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085640

(2-(9-Phenyl-nonylsulfanyl)-5-pyridin-3-ylmethyl-1H...)Show InChI InChI=1S/C25H31N3OS/c29-24-23(18-22-15-11-16-26-19-22)20-27-25(28-24)30-17-10-5-3-1-2-4-7-12-21-13-8-6-9-14-21/h6,8-9,11,13-16,19-20H,1-5,7,10,12,17-18H2,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085652

(2-(8-Phenyl-octylsulfanyl)-5-pyridin-4-ylmethyl-1H...)Show InChI InChI=1S/C24H29N3OS/c28-23-22(18-21-13-15-25-16-14-21)19-26-24(27-23)29-17-9-4-2-1-3-6-10-20-11-7-5-8-12-20/h5,7-8,11-16,19H,1-4,6,9-10,17-18H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085648

(2-[8-(4-Chloro-phenyl)-8-oxo-octylsulfanyl]-5-pyra...)Show SMILES Clc1ccc(cc1)C(=O)CCCCCCCSc1ncc(Cc2cnccn2)c(=O)[nH]1 Show InChI InChI=1S/C23H25ClN4O2S/c24-19-9-7-17(8-10-19)21(29)6-4-2-1-3-5-13-31-23-27-15-18(22(30)28-23)14-20-16-25-11-12-26-20/h7-12,15-16H,1-6,13-14H2,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085651
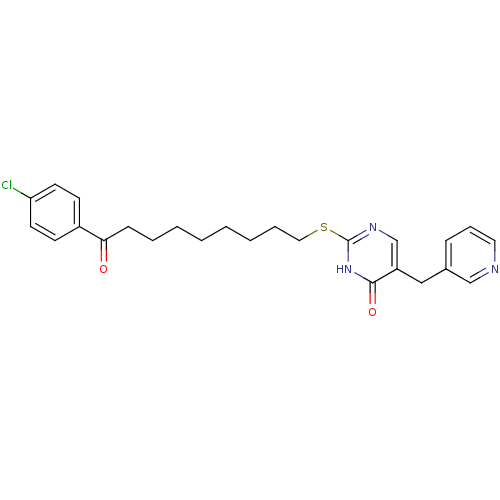
(2-[9-(4-Chloro-phenyl)-9-oxo-nonylsulfanyl]-5-pyri...)Show SMILES Clc1ccc(cc1)C(=O)CCCCCCCCSc1ncc(Cc2cccnc2)c(=O)[nH]1 Show InChI InChI=1S/C25H28ClN3O2S/c26-22-12-10-20(11-13-22)23(30)9-5-3-1-2-4-6-15-32-25-28-18-21(24(31)29-25)16-19-8-7-14-27-17-19/h7-8,10-14,17-18H,1-6,9,15-16H2,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085624

(5-(6-Methyl-pyridin-3-ylmethyl)-2-(8-phenyl-octyls...)Show SMILES Cc1ccc(Cc2cnc(SCCCCCCCCc3ccccc3)[nH]c2=O)cn1 Show InChI InChI=1S/C25H31N3OS/c1-20-14-15-22(18-26-20)17-23-19-27-25(28-24(23)29)30-16-10-5-3-2-4-7-11-21-12-8-6-9-13-21/h6,8-9,12-15,18-19H,2-5,7,10-11,16-17H2,1H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085633

(5-(1-Butyl-2-oxo-1,2-dihydro-pyridin-4-ylmethyl)-2...)Show SMILES CCCCn1ccc(Cc2cnc(SCCCCCCCCc3ccccc3)[nH]c2=O)cc1=O Show InChI InChI=1S/C28H37N3O2S/c1-2-3-17-31-18-16-24(21-26(31)32)20-25-22-29-28(30-27(25)33)34-19-12-7-5-4-6-9-13-23-14-10-8-11-15-23/h8,10-11,14-16,18,21-22H,2-7,9,12-13,17,19-20H2,1H3,(H,29,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085654

(2-(4-Fluoro-benzylsulfanyl)-5-pyrimidin-5-ylmethyl...)Show InChI InChI=1S/C16H13FN4OS/c17-14-3-1-11(2-4-14)9-23-16-20-8-13(15(22)21-16)5-12-6-18-10-19-7-12/h1-4,6-8,10H,5,9H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085631
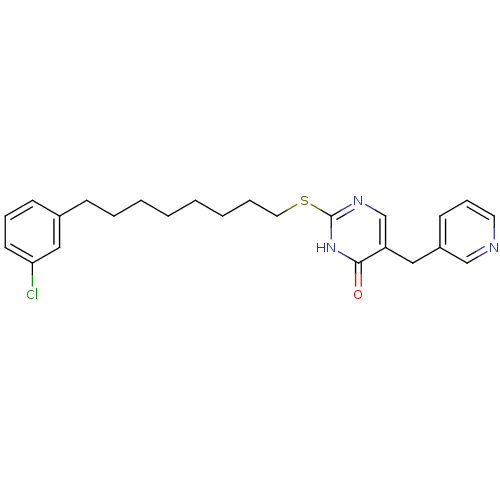
(2-[8-(3-Chloro-phenyl)-octylsulfanyl]-5-pyridin-3-...)Show SMILES Clc1cccc(CCCCCCCCSc2ncc(Cc3cccnc3)c(=O)[nH]2)c1 Show InChI InChI=1S/C24H28ClN3OS/c25-22-12-7-10-19(16-22)9-5-3-1-2-4-6-14-30-24-27-18-21(23(29)28-24)15-20-11-8-13-26-17-20/h7-8,10-13,16-18H,1-6,9,14-15H2,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085643

(5-(6-Methyl-pyridin-3-ylmethyl)-2-(9-phenyl-nonyls...)Show SMILES Cc1ccc(Cc2cnc(SCCCCCCCCCc3ccccc3)[nH]c2=O)cn1 Show InChI InChI=1S/C26H33N3OS/c1-21-15-16-23(19-27-21)18-24-20-28-26(29-25(24)30)31-17-11-6-4-2-3-5-8-12-22-13-9-7-10-14-22/h7,9-10,13-16,19-20H,2-6,8,11-12,17-18H2,1H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085641

(5-(2-Oxo-1,2-dihydro-pyridin-4-ylmethyl)-2-(8-phen...)Show SMILES O=c1cc(Cc2cnc(SCCCCCCCCc3ccccc3)[nH]c2=O)cc[nH]1 Show InChI InChI=1S/C24H29N3O2S/c28-22-17-20(13-14-25-22)16-21-18-26-24(27-23(21)29)30-15-9-4-2-1-3-6-10-19-11-7-5-8-12-19/h5,7-8,11-14,17-18H,1-4,6,9-10,15-16H2,(H,25,28)(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085623

(5-(6-Methyl-pyridin-3-ylmethyl)-2-(7-phenyl-heptyl...)Show SMILES Cc1ccc(Cc2cnc(SCCCCCCCc3ccccc3)[nH]c2=O)cn1 Show InChI InChI=1S/C24H29N3OS/c1-19-13-14-21(17-25-19)16-22-18-26-24(27-23(22)28)29-15-9-4-2-3-6-10-20-11-7-5-8-12-20/h5,7-8,11-14,17-18H,2-4,6,9-10,15-16H2,1H3,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085645

(5-(2-Methoxy-pyridin-4-ylmethyl)-2-(8-phenyl-octyl...)Show SMILES COc1cc(Cc2cnc(SCCCCCCCCc3ccccc3)[nH]c2=O)ccn1 Show InChI InChI=1S/C25H31N3O2S/c1-30-23-18-21(14-15-26-23)17-22-19-27-25(28-24(22)29)31-16-10-5-3-2-4-7-11-20-12-8-6-9-13-20/h6,8-9,12-15,18-19H,2-5,7,10-11,16-17H2,1H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085627

(2-(7-Phenyl-heptylsulfanyl)-5-pyridin-3-ylmethyl-1...)Show InChI InChI=1S/C23H27N3OS/c27-22-21(16-20-13-9-14-24-17-20)18-25-23(26-22)28-15-8-3-1-2-5-10-19-11-6-4-7-12-19/h4,6-7,9,11-14,17-18H,1-3,5,8,10,15-16H2,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085630

(2-Benzylsulfanyl-5-pyridin-3-ylmethyl-1H-pyrimidin...)Show InChI InChI=1S/C17H15N3OS/c21-16-15(9-14-7-4-8-18-10-14)11-19-17(20-16)22-12-13-5-2-1-3-6-13/h1-8,10-11H,9,12H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085650
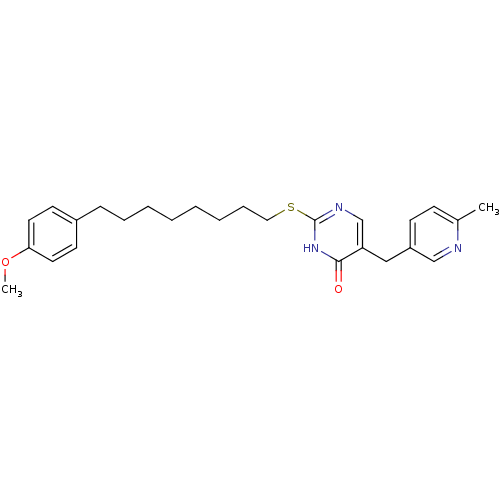
(2-[8-(4-Methoxy-phenyl)-octylsulfanyl]-5-(6-methyl...)Show SMILES COc1ccc(CCCCCCCCSc2ncc(Cc3ccc(C)nc3)c(=O)[nH]2)cc1 Show InChI InChI=1S/C26H33N3O2S/c1-20-10-11-22(18-27-20)17-23-19-28-26(29-25(23)30)32-16-8-6-4-3-5-7-9-21-12-14-24(31-2)15-13-21/h10-15,18-19H,3-9,16-17H2,1-2H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085649

(2-Benzylsulfanyl-5-pyrimidin-5-ylmethyl-1H-pyrimid...)Show InChI InChI=1S/C16H14N4OS/c21-15-14(6-13-7-17-11-18-8-13)9-19-16(20-15)22-10-12-4-2-1-3-5-12/h1-5,7-9,11H,6,10H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085647

(5-(6-Methyl-pyridin-3-ylmethyl)-2-(6-phenyl-hexyls...)Show InChI InChI=1S/C23H27N3OS/c1-18-12-13-20(16-24-18)15-21-17-25-23(26-22(21)27)28-14-8-3-2-5-9-19-10-6-4-7-11-19/h4,6-7,10-13,16-17H,2-3,5,8-9,14-15H2,1H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085621

(2-[6-(4-Chloro-benzyloxy)-hexylsulfanyl]-5-(6-meth...)Show SMILES Cc1ccc(Cc2cnc(SCCCCCCOCc3ccc(Cl)cc3)[nH]c2=O)cn1 Show InChI InChI=1S/C24H28ClN3O2S/c1-18-6-7-20(15-26-18)14-21-16-27-24(28-23(21)29)31-13-5-3-2-4-12-30-17-19-8-10-22(25)11-9-19/h6-11,15-16H,2-5,12-14,17H2,1H3,(H,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085639

(2-(8-Oxo-8-thiophen-2-yl-octylsulfanyl)-5-pyridin-...)Show SMILES O=C(CCCCCCCSc1ncc(Cc2cccnc2)c(=O)[nH]1)c1cccs1 Show InChI InChI=1S/C22H25N3O2S2/c26-19(20-10-7-13-28-20)9-4-2-1-3-5-12-29-22-24-16-18(21(27)25-22)14-17-8-6-11-23-15-17/h6-8,10-11,13,15-16H,1-5,9,12,14H2,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085617
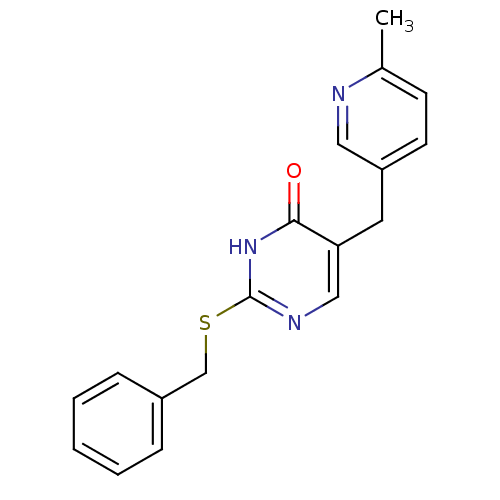
(2-Benzylsulfanyl-5-(6-methyl-pyridin-3-ylmethyl)-1...)Show InChI InChI=1S/C18H17N3OS/c1-13-7-8-15(10-19-13)9-16-11-20-18(21-17(16)22)23-12-14-5-3-2-4-6-14/h2-8,10-11H,9,12H2,1H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085619

(2-(6-Phenyl-hexylsulfanyl)-5-pyridin-3-ylmethyl-1H...)Show InChI InChI=1S/C22H25N3OS/c26-21-20(15-19-12-8-13-23-16-19)17-24-22(25-21)27-14-7-2-1-4-9-18-10-5-3-6-11-18/h3,5-6,8,10-13,16-17H,1-2,4,7,9,14-15H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085646

(2-[8-(4-Acetyl-phenyl)-octylsulfanyl]-5-pyridin-3-...)Show SMILES CC(=O)c1ccc(CCCCCCCCSc2ncc(Cc3cccnc3)c(=O)[nH]2)cc1 Show InChI InChI=1S/C26H31N3O2S/c1-20(30)23-13-11-21(12-14-23)9-6-4-2-3-5-7-16-32-26-28-19-24(25(31)29-26)17-22-10-8-15-27-18-22/h8,10-15,18-19H,2-7,9,16-17H2,1H3,(H,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085635
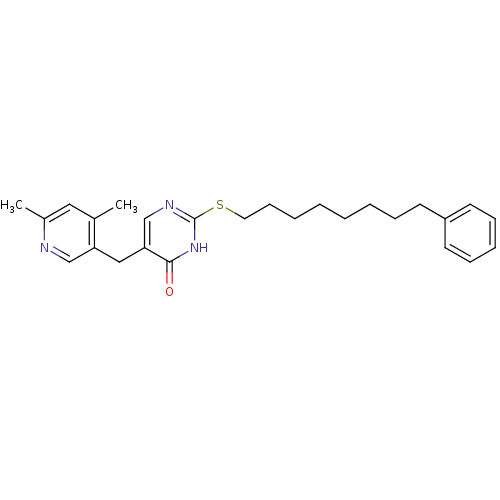
(5-(4,6-Dimethyl-pyridin-3-ylmethyl)-2-(8-phenyl-oc...)Show SMILES Cc1cc(C)c(Cc2cnc(SCCCCCCCCc3ccccc3)[nH]c2=O)cn1 Show InChI InChI=1S/C26H33N3OS/c1-20-16-21(2)27-18-23(20)17-24-19-28-26(29-25(24)30)31-15-11-6-4-3-5-8-12-22-13-9-7-10-14-22/h7,9-10,13-14,16,18-19H,3-6,8,11-12,15,17H2,1-2H3,(H,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085638

(2-(4-Chloro-benzylsulfanyl)-5-pyridin-3-ylmethyl-1...)Show InChI InChI=1S/C17H14ClN3OS/c18-15-5-3-12(4-6-15)11-23-17-20-10-14(16(22)21-17)8-13-2-1-7-19-9-13/h1-7,9-10H,8,11H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085637

(2-Benzylsulfanyl-5-pyridin-4-ylmethyl-1H-pyrimidin...)Show InChI InChI=1S/C17H15N3OS/c21-16-15(10-13-6-8-18-9-7-13)11-19-17(20-16)22-12-14-4-2-1-3-5-14/h1-9,11H,10,12H2,(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085636

(5-(6-Methyl-pyridin-3-ylmethyl)-2-phenethylsulfany...)Show InChI InChI=1S/C19H19N3OS/c1-14-7-8-16(12-20-14)11-17-13-21-19(22-18(17)23)24-10-9-15-5-3-2-4-6-15/h2-8,12-13H,9-11H2,1H3,(H,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085632
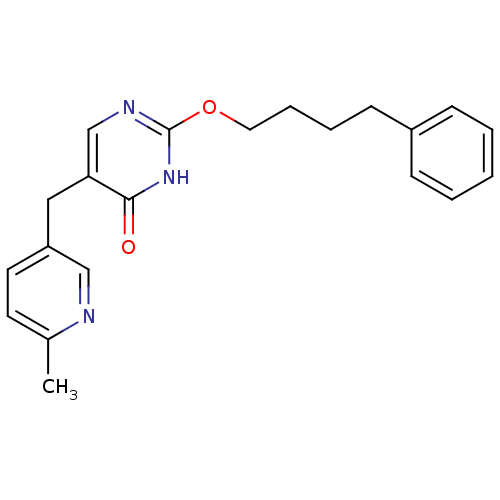
(5-(6-Methyl-pyridin-3-ylmethyl)-2-(4-phenyl-butoxy...)Show InChI InChI=1S/C21H23N3O2/c1-16-10-11-18(14-22-16)13-19-15-23-21(24-20(19)25)26-12-6-5-9-17-7-3-2-4-8-17/h2-4,7-8,10-11,14-15H,5-6,9,12-13H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085644
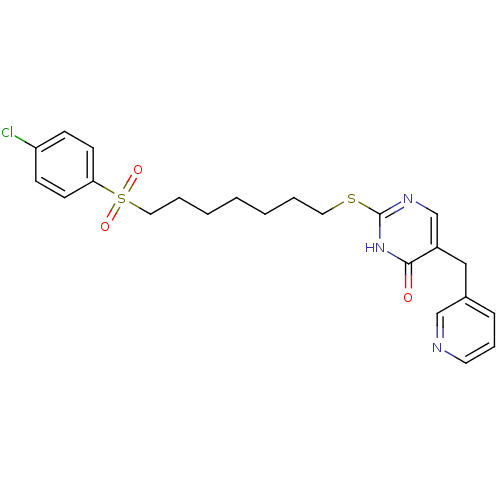
(2-[7-(4-Chloro-benzenesulfonyl)-heptylsulfanyl]-5-...)Show SMILES Clc1ccc(cc1)S(=O)(=O)CCCCCCCSc1ncc(Cc2cccnc2)c(=O)[nH]1 Show InChI InChI=1S/C23H26ClN3O3S2/c24-20-8-10-21(11-9-20)32(29,30)14-5-3-1-2-4-13-31-23-26-17-19(22(28)27-23)15-18-7-6-12-25-16-18/h6-12,16-17H,1-5,13-15H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
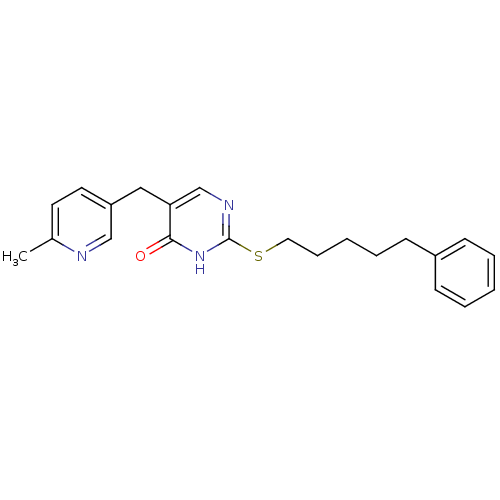
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085642
(5-(6-Methyl-pyridin-3-ylmethyl)-2-(5-phenyl-pentyl...)Show InChI InChI=1S/C22H25N3OS/c1-17-11-12-19(15-23-17)14-20-16-24-22(25-21(20)26)27-13-7-3-6-10-18-8-4-2-5-9-18/h2,4-5,8-9,11-12,15-16H,3,6-7,10,13-14H2,1H3,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
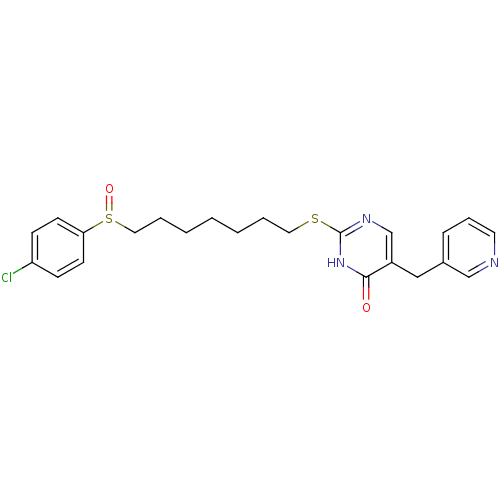
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085653
(2-[7-(4-Chloro-benzenesulfinyl)-heptylsulfanyl]-5-...)Show SMILES Clc1ccc(cc1)S(=O)CCCCCCCSc1ncc(Cc2cccnc2)c(=O)[nH]1 Show InChI InChI=1S/C23H26ClN3O2S2/c24-20-8-10-21(11-9-20)31(29)14-5-3-1-2-4-13-30-23-26-17-19(22(28)27-23)15-18-7-6-12-25-16-18/h6-12,16-17H,1-5,13-15H2,(H,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |
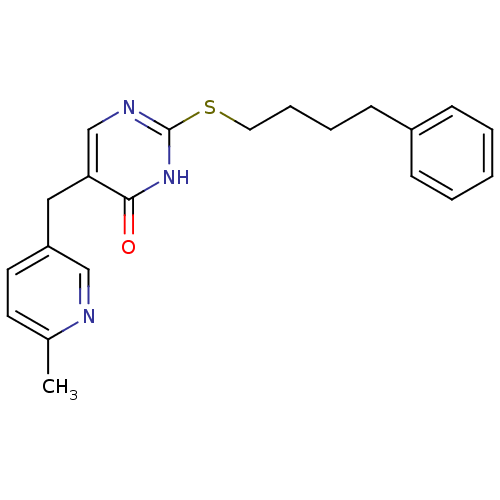
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50085618
(5-(6-Methyl-pyridin-3-ylmethyl)-2-(4-phenyl-butyls...)Show InChI InChI=1S/C21H23N3OS/c1-16-10-11-18(14-22-16)13-19-15-23-21(24-20(19)25)26-12-6-5-9-17-7-3-2-4-8-17/h2-4,7-8,10-11,14-15H,5-6,9,12-13H2,1H3,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of lipoprotein-associated phospholipase A2 (Lp-PLA2) |
Bioorg Med Chem Lett 10: 395-8 (2000)
BindingDB Entry DOI: 10.7270/Q25X285S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data