 Found 63 hits of Enzyme Inhibition Constant Data
Found 63 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086557
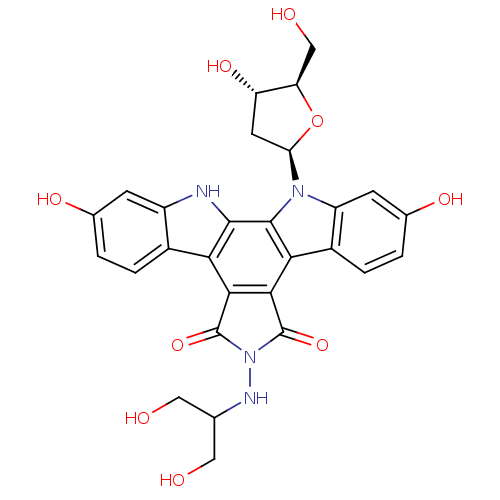
(CHEMBL334337 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3C[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O9/c33-8-11(9-34)30-32-27(39)23-21-14-3-1-12(36)5-16(14)29-25(21)26-22(24(23)28(32)40)15-4-2-13(37)6-17(15)31(26)20-7-18(38)19(10-35)41-20/h1-6,11,18-20,29-30,33-38H,7-10H2/t18-,19+,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086562
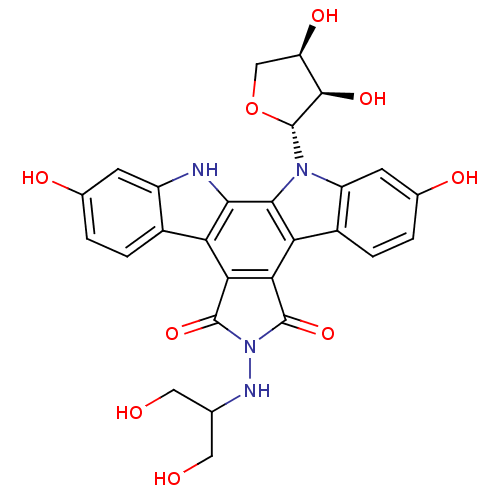
(CHEMBL334360 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3OC[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C27H24N4O9/c32-7-10(8-33)29-31-25(38)20-18-13-3-1-11(34)5-15(13)28-22(18)23-19(21(20)26(31)39)14-4-2-12(35)6-16(14)30(23)27-24(37)17(36)9-40-27/h1-6,10,17,24,27-29,32-37H,7-9H2/t17-,24-,27-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086560

(CHEMBL338966 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25-,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086563
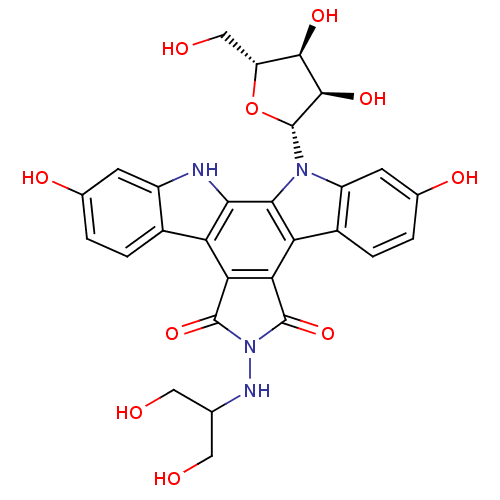
(CHEMBL135492 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086558
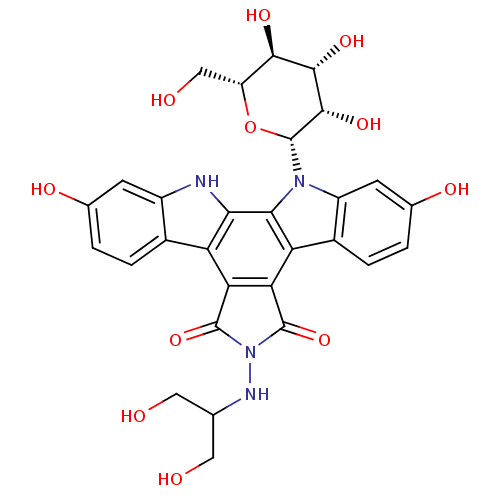
(CHEMBL406901 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26+,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086559
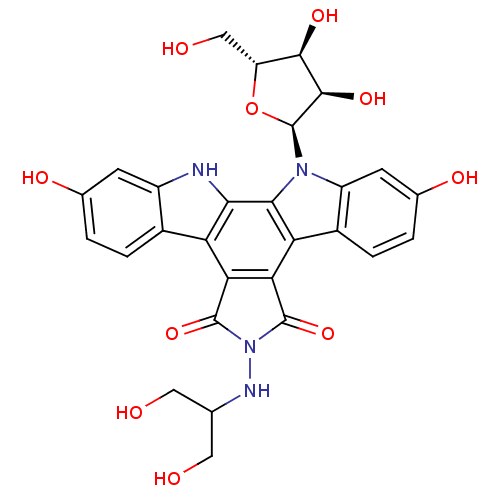
(CHEMBL135970 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086572

(CHEMBL434237 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086564

(CHEMBL335940 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26+,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086561

(CHEMBL134536 | NB-506 Analogue)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1c2cc(O)ccc2c2c3C(=O)N(NC(CO)CO)C(=O)c3c3c4ccc(O)cc4[nH]c3c12 Show InChI InChI=1S/C29H28N4O10/c1-10-24(38)25(39)26(40)29(43-10)32-17-7-13(37)3-5-15(17)19-21-20(27(41)33(28(21)42)31-11(8-34)9-35)18-14-4-2-12(36)6-16(14)30-22(18)23(19)32/h2-7,10-11,24-26,29-31,34-40H,8-9H2,1H3/t10-,24-,25+,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086576

(CHEMBL334869 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25-,26-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086575
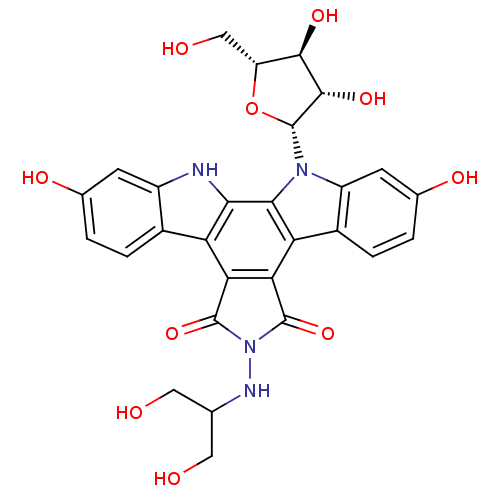
(CHEMBL132533 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086555

(CHEMBL405405 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3C[C@@H](O)[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O10/c34-8-11(9-35)31-33-28(41)23-21-14-3-1-12(37)5-16(14)30-25(21)26-22(24(23)29(33)42)15-4-2-13(38)6-17(15)32(26)20-7-18(39)27(40)19(10-36)43-20/h1-6,11,18-20,27,30-31,34-40H,7-10H2/t18-,19-,20-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086556

(CHEMBL334461 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3C[C@@H](O)[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O10/c34-8-11(9-35)31-33-28(41)23-21-14-3-1-12(37)5-16(14)30-25(21)26-22(24(23)29(33)42)15-4-2-13(38)6-17(15)32(26)20-7-18(39)27(40)19(10-36)43-20/h1-6,11,18-20,27,30-31,34-40H,7-10H2/t18-,19-,20+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086565

(CHEMBL135881 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@@H](C(O)CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-25(41)24(40)26(44-29)17(39)9-36/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17?,24-,25+,26-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086571
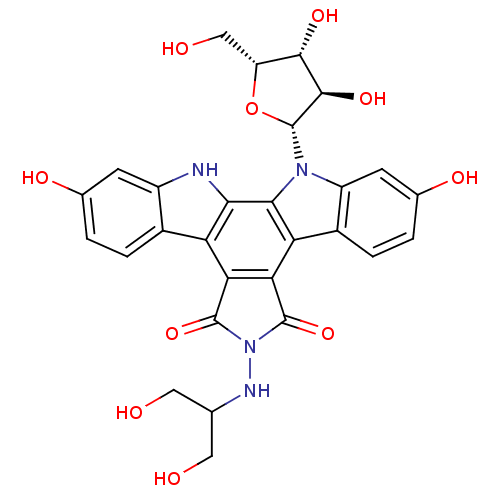
(CHEMBL135491 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086568
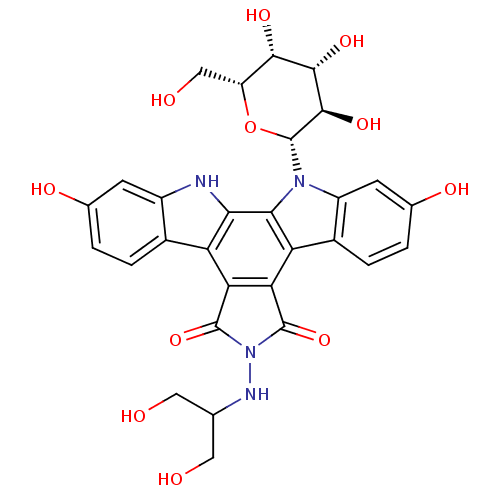
(CHEMBL336603 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24+,25+,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086574

(CHEMBL433855 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25+,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50403823
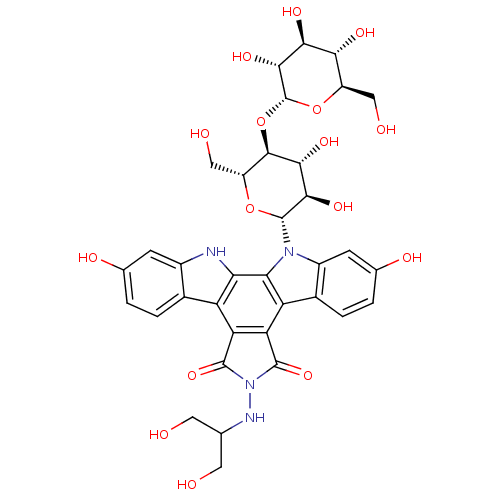
(CHEMBL2029060)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O[C@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C35H38N4O16/c40-7-11(8-41)37-39-32(51)22-20-14-3-1-12(44)5-16(14)36-24(20)25-21(23(22)33(39)52)15-4-2-13(45)6-17(15)38(25)34-29(49)28(48)31(19(10-43)53-34)55-35-30(50)27(47)26(46)18(9-42)54-35/h1-6,11,18-19,26-31,34-37,40-50H,7-10H2/t18-,19-,26-,27+,28-,29-,30-,31-,34-,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086566
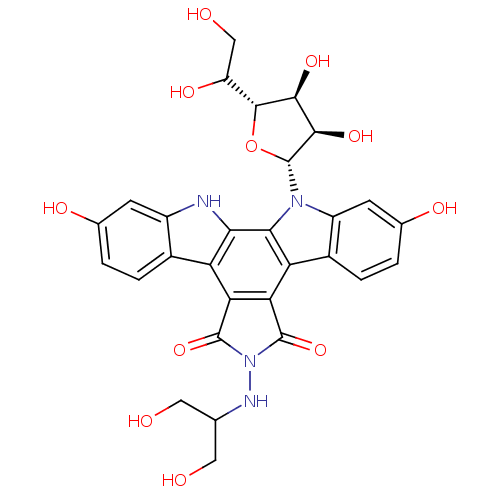
(CHEMBL336626 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](C(O)CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-25(41)24(40)26(44-29)17(39)9-36/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17?,24-,25+,26+,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086567

(CHEMBL133209 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086569

(CHEMBL338967 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(35)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(36)6-16(14)31(23)28-25(39)24(38)17(37)9-42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM50086570

(CHEMBL435191 | Edotecarin | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on protein kinase C using histone II-As as substrate |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086556

(CHEMBL334461 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3C[C@@H](O)[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O10/c34-8-11(9-35)31-33-28(41)23-21-14-3-1-12(37)5-16(14)30-25(21)26-22(24(23)29(33)42)15-4-2-13(38)6-17(15)32(26)20-7-18(39)27(40)19(10-36)43-20/h1-6,11,18-20,27,30-31,34-40H,7-10H2/t18-,19-,20+,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086575

(CHEMBL132533 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25+,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086571

(CHEMBL135491 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086555

(CHEMBL405405 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3C[C@@H](O)[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O10/c34-8-11(9-35)31-33-28(41)23-21-14-3-1-12(37)5-16(14)30-25(21)26-22(24(23)29(33)42)15-4-2-13(38)6-17(15)32(26)20-7-18(39)27(40)19(10-36)43-20/h1-6,11,18-20,27,30-31,34-40H,7-10H2/t18-,19-,20-,27+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086563

(CHEMBL135492 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086561

(CHEMBL134536 | NB-506 Analogue)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1c2cc(O)ccc2c2c3C(=O)N(NC(CO)CO)C(=O)c3c3c4ccc(O)cc4[nH]c3c12 Show InChI InChI=1S/C29H28N4O10/c1-10-24(38)25(39)26(40)29(43-10)32-17-7-13(37)3-5-15(17)19-21-20(27(41)33(28(21)42)31-11(8-34)9-35)18-14-4-2-12(36)6-16(14)30-22(18)23(19)32/h2-7,10-11,24-26,29-31,34-40H,8-9H2,1H3/t10-,24-,25+,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50403823

(CHEMBL2029060)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O[C@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C35H38N4O16/c40-7-11(8-41)37-39-32(51)22-20-14-3-1-12(44)5-16(14)36-24(20)25-21(23(22)33(39)52)15-4-2-13(45)6-17(15)38(25)34-29(49)28(48)31(19(10-43)53-34)55-35-30(50)27(47)26(46)18(9-42)54-35/h1-6,11,18-19,26-31,34-37,40-50H,7-10H2/t18-,19-,26-,27+,28-,29-,30-,31-,34-,35-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086557

(CHEMBL334337 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3C[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O9/c33-8-11(9-34)30-32-27(39)23-21-14-3-1-12(36)5-16(14)29-25(21)26-22(24(23)28(32)40)15-4-2-13(37)6-17(15)31(26)20-7-18(38)19(10-35)41-20/h1-6,11,18-20,29-30,33-38H,7-10H2/t18-,19+,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086558

(CHEMBL406901 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26+,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086569

(CHEMBL338967 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(35)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(36)6-16(14)31(23)28-25(39)24(38)17(37)9-42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086576

(CHEMBL334869 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25-,26-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086562

(CHEMBL334360 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3OC[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C27H24N4O9/c32-7-10(8-33)29-31-25(38)20-18-13-3-1-11(34)5-15(13)28-22(18)23-19(21(20)26(31)39)14-4-2-12(35)6-16(14)30(23)27-24(37)17(36)9-40-27/h1-6,10,17,24,27-29,32-37H,7-9H2/t17-,24-,27-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086555

(CHEMBL405405 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3C[C@@H](O)[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O10/c34-8-11(9-35)31-33-28(41)23-21-14-3-1-12(37)5-16(14)30-25(21)26-22(24(23)29(33)42)15-4-2-13(38)6-17(15)32(26)20-7-18(39)27(40)19(10-36)43-20/h1-6,11,18-20,27,30-31,34-40H,7-10H2/t18-,19-,20-,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 240 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086557

(CHEMBL334337 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3C[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O9/c33-8-11(9-34)30-32-27(39)23-21-14-3-1-12(36)5-16(14)29-25(21)26-22(24(23)28(32)40)15-4-2-13(37)6-17(15)31(26)20-7-18(38)19(10-35)41-20/h1-6,11,18-20,29-30,33-38H,7-10H2/t18-,19+,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086565

(CHEMBL135881 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@@H](C(O)CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-25(41)24(40)26(44-29)17(39)9-36/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17?,24-,25+,26-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086566

(CHEMBL336626 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](C(O)CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-25(41)24(40)26(44-29)17(39)9-36/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17?,24-,25+,26+,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086560

(CHEMBL338966 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25-,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086572

(CHEMBL434237 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26-,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086575

(CHEMBL132533 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25+,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086564

(CHEMBL335940 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26+,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086564

(CHEMBL335940 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086570

(CHEMBL435191 | Edotecarin | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086571

(CHEMBL135491 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086574

(CHEMBL433855 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25+,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086558

(CHEMBL406901 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26+,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086556

(CHEMBL334461 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3C[C@@H](O)[C@H](O)[C@@H](CO)O3)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O10/c34-8-11(9-35)31-33-28(41)23-21-14-3-1-12(37)5-16(14)30-25(21)26-22(24(23)29(33)42)15-4-2-13(38)6-17(15)32(26)20-7-18(39)27(40)19(10-36)43-20/h1-6,11,18-20,27,30-31,34-40H,7-10H2/t18-,19-,20+,27+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 130 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086560

(CHEMBL338966 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25-,26-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086561

(CHEMBL134536 | NB-506 Analogue)Show SMILES C[C@H]1O[C@H]([C@H](O)[C@@H](O)[C@@H]1O)n1c2cc(O)ccc2c2c3C(=O)N(NC(CO)CO)C(=O)c3c3c4ccc(O)cc4[nH]c3c12 Show InChI InChI=1S/C29H28N4O10/c1-10-24(38)25(39)26(40)29(43-10)32-17-7-13(37)3-5-15(17)19-21-20(27(41)33(28(21)42)31-11(8-34)9-35)18-14-4-2-12(36)6-16(14)30-22(18)23(19)32/h2-7,10-11,24-26,29-31,34-40H,8-9H2,1H3/t10-,24-,25+,26-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086567

(CHEMBL133209 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086570

(CHEMBL435191 | Edotecarin | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086565

(CHEMBL135881 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@@H](C(O)CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-25(41)24(40)26(44-29)17(39)9-36/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17?,24-,25+,26-,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086563

(CHEMBL135492 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50403823

(CHEMBL2029060)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@@H](O[C@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C35H38N4O16/c40-7-11(8-41)37-39-32(51)22-20-14-3-1-12(44)5-16(14)36-24(20)25-21(23(22)33(39)52)15-4-2-13(45)6-17(15)38(25)34-29(49)28(48)31(19(10-43)53-34)55-35-30(50)27(47)26(46)18(9-42)54-35/h1-6,11,18-19,26-31,34-37,40-50H,7-10H2/t18-,19-,26-,27+,28-,29-,30-,31-,34-,35-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086572

(CHEMBL434237 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24-,25+,26-,29+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086574

(CHEMBL433855 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25+,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086568

(CHEMBL336603 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](CO)[C@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-26(41)25(40)24(39)17(9-36)44-29/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17-,24+,25+,26-,29-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086559

(CHEMBL135970 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086569

(CHEMBL338967 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3OC[C@@H](O)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(35)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(36)6-16(14)31(23)28-25(39)24(38)17(37)9-42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086567

(CHEMBL133209 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24+,25-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 1
(Homo sapiens (Human)) | BDBM50086566

(CHEMBL336626 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@@H]3O[C@H](C(O)CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C29H28N4O11/c34-7-10(8-35)31-33-27(42)20-18-13-3-1-11(37)5-15(13)30-22(18)23-19(21(20)28(33)43)14-4-2-12(38)6-16(14)32(23)29-25(41)24(40)26(44-29)17(39)9-36/h1-6,10,17,24-26,29-31,34-41H,7-9H2/t17?,24-,25+,26+,29+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory effect on topoisomerase-I mediated DNA cleavage using supercoiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |
DNA topoisomerase 2-alpha/2-beta
(Homo sapiens (Human)) | BDBM50086559

(CHEMBL135970 | NB-506 Analogue)Show SMILES OCC(CO)NN1C(=O)c2c(C1=O)c1c3ccc(O)cc3n([C@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c1c1[nH]c3cc(O)ccc3c21 Show InChI InChI=1S/C28H26N4O10/c33-7-10(8-34)30-32-26(40)20-18-13-3-1-11(36)5-15(13)29-22(18)23-19(21(20)27(32)41)14-4-2-12(37)6-16(14)31(23)28-25(39)24(38)17(9-35)42-28/h1-6,10,17,24-25,28-30,33-39H,7-9H2/t17-,24-,25-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute
Curated by ChEMBL
| Assay Description
Tested for inhibitory effect on topoisomerase-2 mediated DNA cleavage using super coiled pBR322 plasmid DNA |
Bioorg Med Chem Lett 10: 419-22 (2000)
BindingDB Entry DOI: 10.7270/Q2F76BSS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data