

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50145605 (4-amino-1-[(2R,5S)-5-(hydroxymethyl)tetrahydrofura...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408884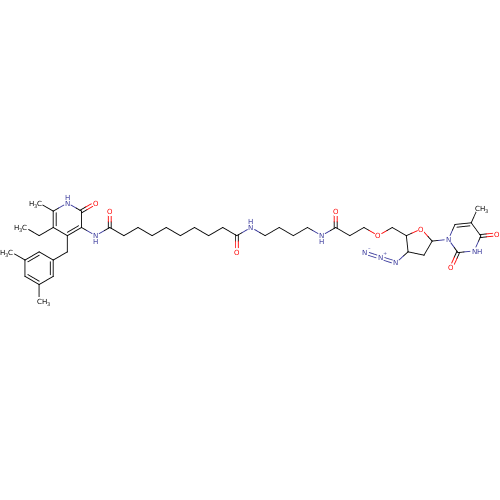 (CHEMBL301685) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408894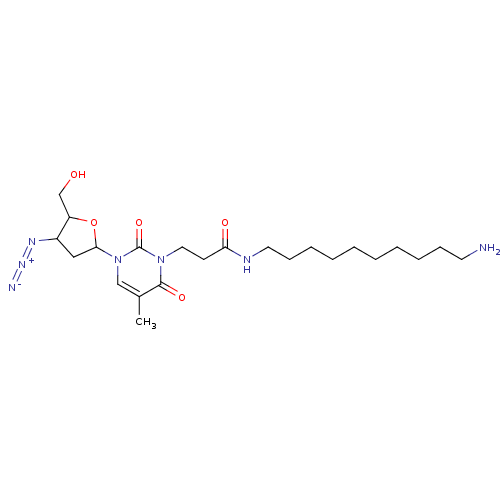 (CHEMBL292392) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.20E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50002692 ((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408885 (CHEMBL57313) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408888 (CHEMBL417227) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408896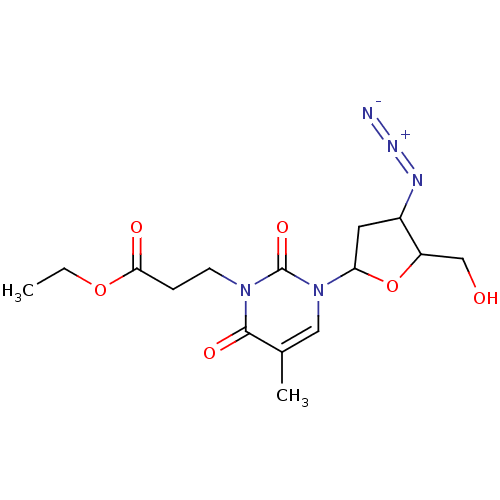 (CHEMBL56328) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 800 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408902 (CHEMBL58653) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408878 (CHEMBL57590) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408879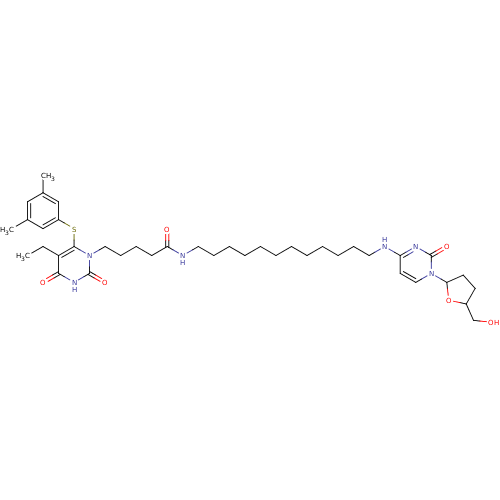 (CHEMBL20610) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 430 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408880 (CHEMBL58213) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408890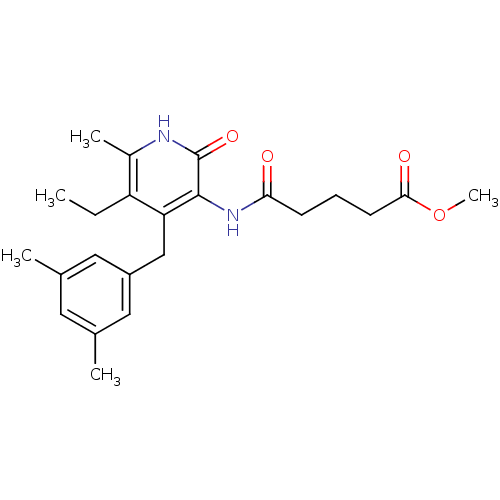 (CHEMBL57301) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408891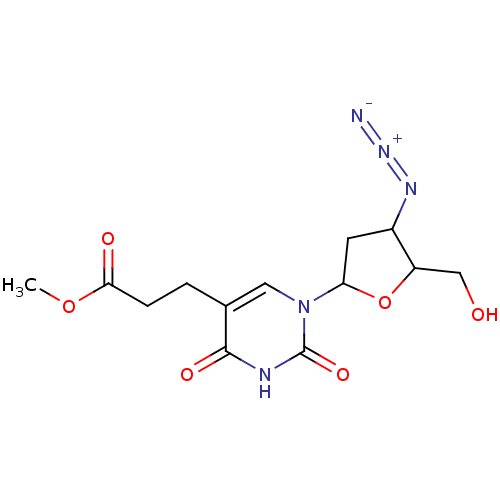 (CHEMBL58891) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408893 (CHEMBL58914) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM10905 (3-Amino-4-(3,5-dimethylbenzyl)-5-ethyl-6-methylpyr...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408899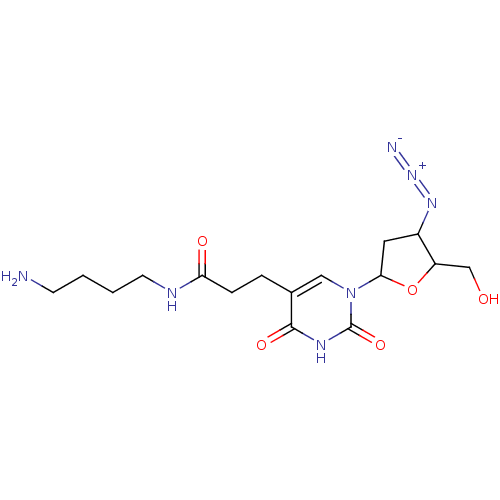 (CHEMBL300607) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408900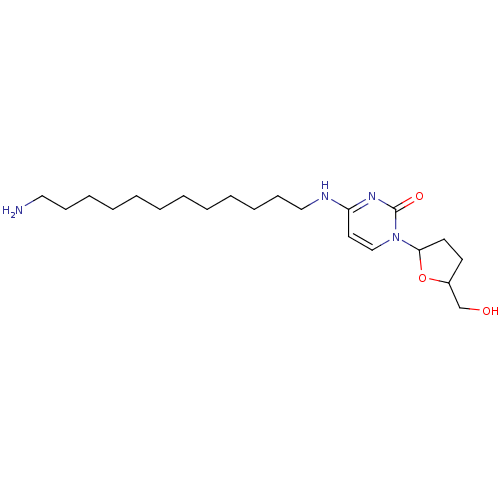 (CHEMBL417953) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408895 (CHEMBL440482) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408901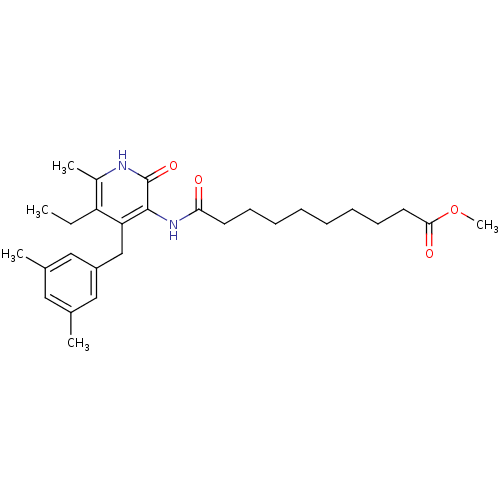 (CHEMBL60873) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408881 (CHEMBL418119) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408883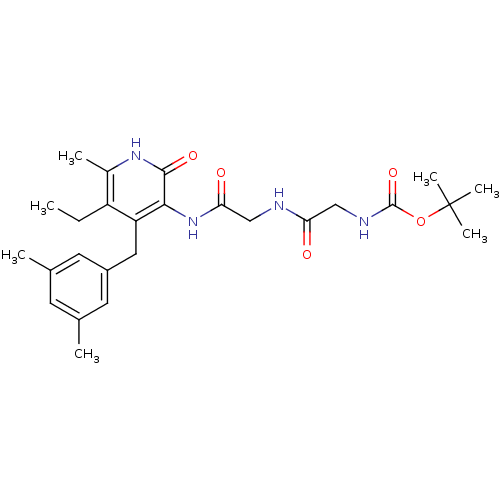 (CHEMBL58497) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408887 (CHEMBL418294) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 7.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408889 (CHEMBL57161) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408897 (CHEMBL262003) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408886 (CHEMBL57929) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408882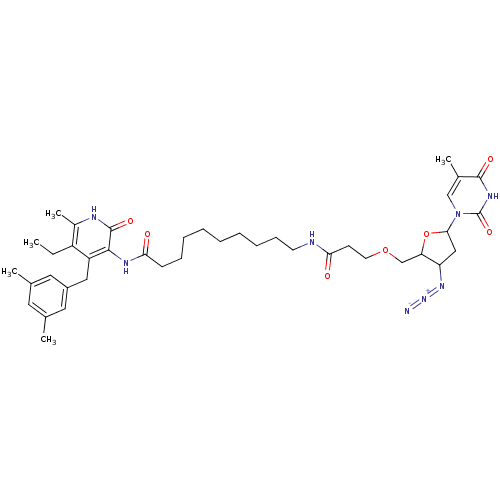 (CHEMBL59514) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50408892 (CHEMBL418118) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
UMR 176 CNRS/Institut Curie Curated by ChEMBL | Assay Description Effective concentration required to achieve 50% inhibition of HIV-1 LAI replication in human T4 lymphoblastoid CEM-SS cells. | J Med Chem 43: 1927-39 (2000) BindingDB Entry DOI: 10.7270/Q2WS8VFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||