

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor (RABBIT) | BDBM50044518 (5-Butyl-2-(2-nitro-phenyl)-4-[2'-(1H-tetrazol-5-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Angiotensin II receptor, type 1 | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035431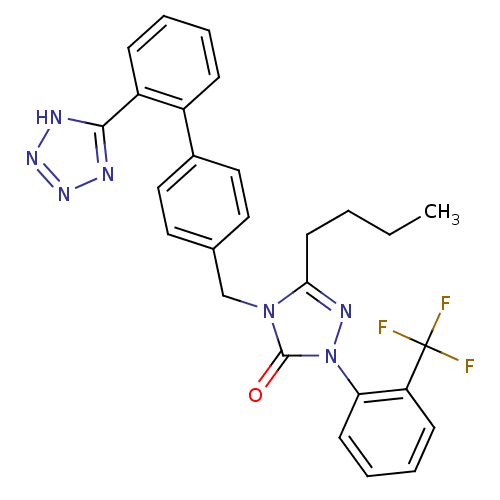 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Angiotensin II receptor, type 1 | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50044543 (5-Butyl-2-(2-isopropyl-phenyl)-4-[2'-(1H-tetrazol-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Angiotensin II receptor, type 1 | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50088244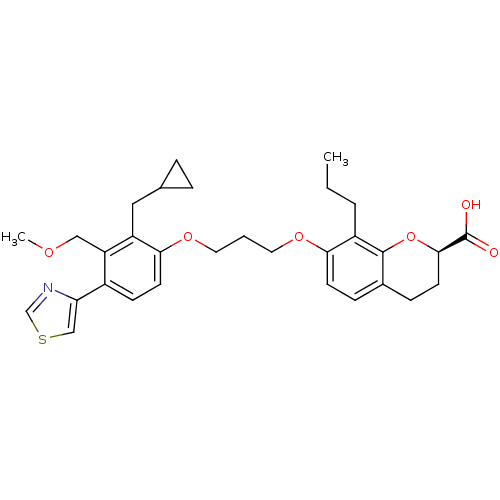 (7-[3-(2-Cyclopropylmethyl-3-methoxymethyl-4-thiazo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to leukotriene B4 receptor | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50088245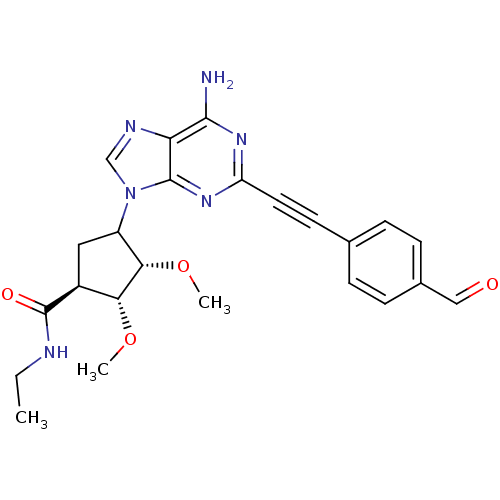 (4-[6-Amino-2-(4-formyl-phenylethynyl)-purin-9-yl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Adenosine A2A receptor | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50035451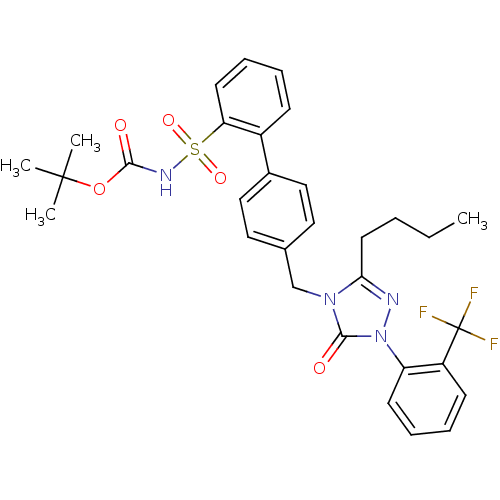 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Angiotensin II receptor, type 2 | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50088242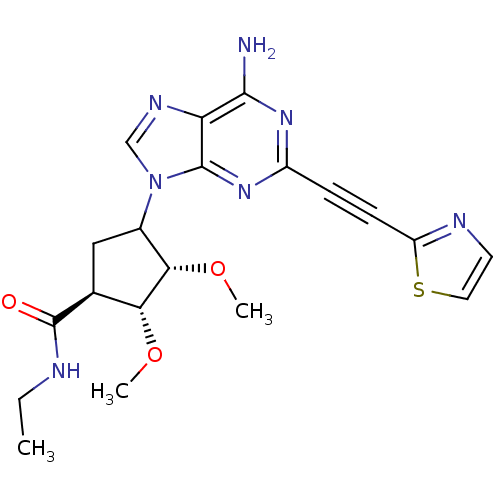 (4-(6-Amino-2-thiazol-2-ylethynyl-purin-9-yl)-2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Adenosine A2A receptor | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50088241 (3-(3,4-Dichloro-phenyl)-5-hydroxy-4-(4-methoxy-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Endothelin A receptor | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50035441 (4'-[3-Butyl-1-(3-nitro-phenyl)-5-oxo-1,5-dihydro-[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Angiotensin II receptor, type 2 | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50088246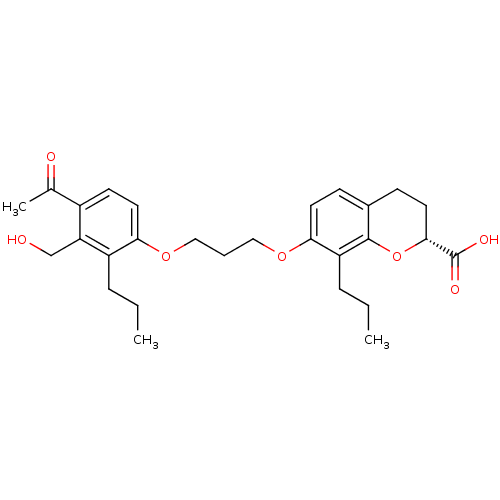 (7-[3-(4-Acetyl-3-hydroxymethyl-2-propyl-phenoxy)-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to leukotriene B4 receptor | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50088243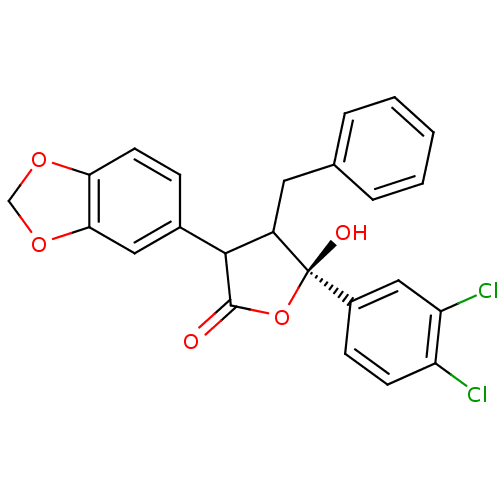 (3-Benzo[1,3]dioxol-5-yl-4-benzyl-5-(3,4-dichloro-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Binding affinity to Endothelin A receptor | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50035451 (2-{4-[3-butyl-5-oxo-1-(2-trifluoromethylphenyl)-4,...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 2 binding | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Sus scrofa) | BDBM50037605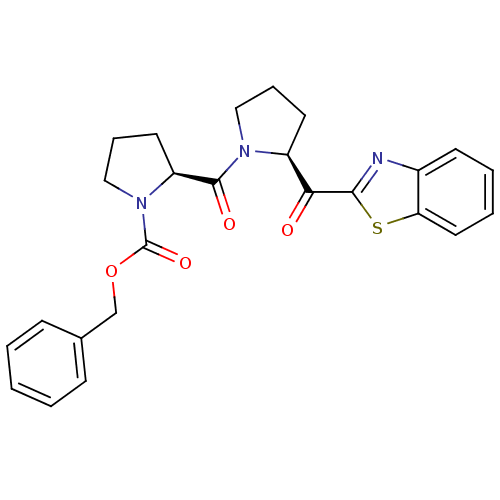 ((S)-2-[(S)-2-(Benzothiazole-2-carbonyl)-pyrrolidin...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase activity | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Sus scrofa) | BDBM50088247 (CHEMBL303966 | Oxo-{(S)-1-[(S)-1-(4-phenyl-butyryl...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibition of Prolyl endopeptidase activity | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50034013 ((S)-9-Bromo-4-ethyl-4-hydroxy-11-methyl-1,12-dihyd...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA topoisomerase I | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (RAT) | BDBM50035441 (4'-[3-Butyl-1-(3-nitro-phenyl)-5-oxo-1,5-dihydro-[...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 2 binding | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50034014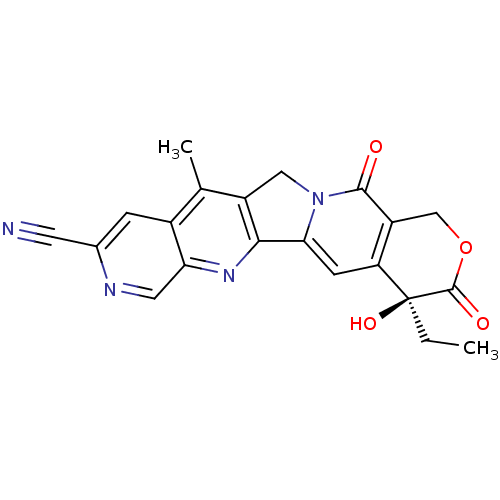 ((S)-4-Ethyl-4-hydroxy-11-methyl-3,13-dioxo-3,4,12,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against DNA topoisomerase I | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (RABBIT) | BDBM50035431 (5-Butyl-4-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmeth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeoGenesis, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Angiotensin II receptor, type 1 binding | J Med Chem 43: 1993-2006 (2000) BindingDB Entry DOI: 10.7270/Q2MP53ZS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||