

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-lactamase (Staphylococcus aureus) | BDBM50033680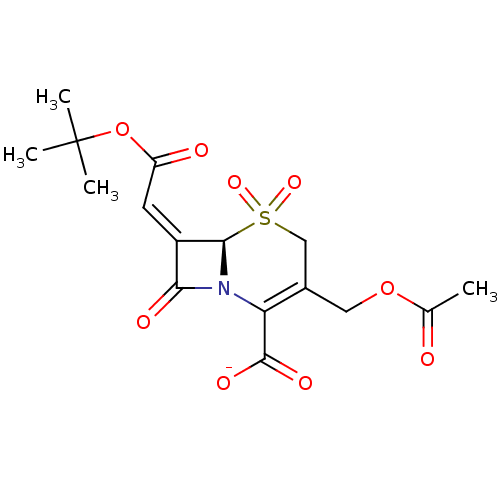 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088813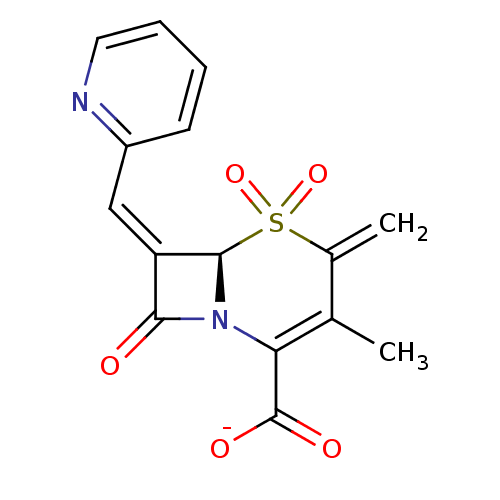 (CHEMBL166730 | Sodium; (R)-3-methyl-4-methylene-5,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088810 (CHEMBL353004 | Sodium; (R)-3-acetoxymethyl-4-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 165 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50157692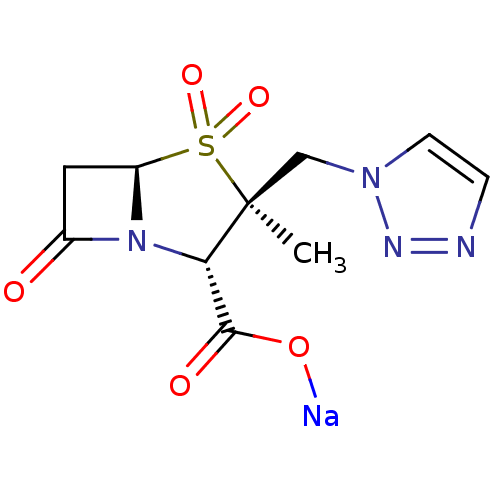 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50033701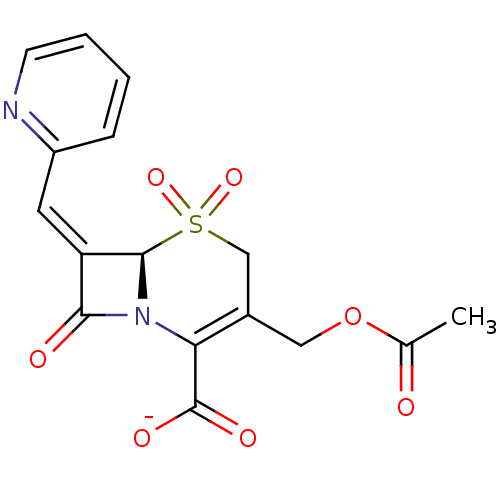 (CHEMBL268632 | Sodium; (R)-3-acetoxymethyl-5,5,8-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088815 (CHEMBL171001 | Sodium; (R)-4-(bis-methylsulfanyl-m...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088814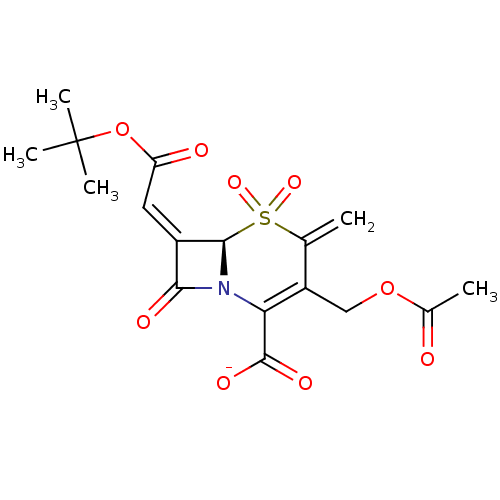 (CHEMBL169515 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 932 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088806 (CHEMBL169016 | Sodium; (R)-7-[1-tert-butoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088814 (CHEMBL169515 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088806 (CHEMBL169016 | Sodium; (R)-7-[1-tert-butoxycarbony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088808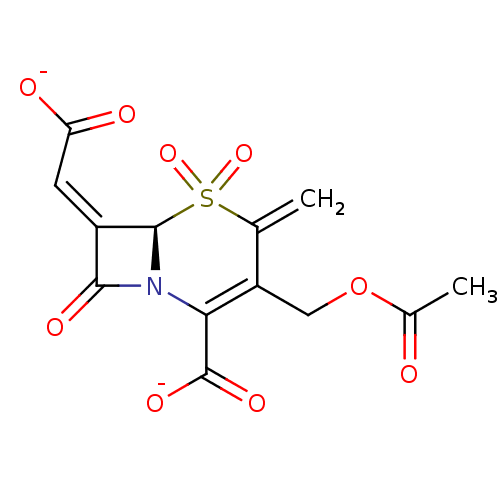 (CHEMBL355571 | [(R)-3-Acetoxymethyl-2-carboxy-4-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50157692 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088807 (CHEMBL435467 | Sodium; (R)-3,4-dimethyl-5,5,8-trio...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088812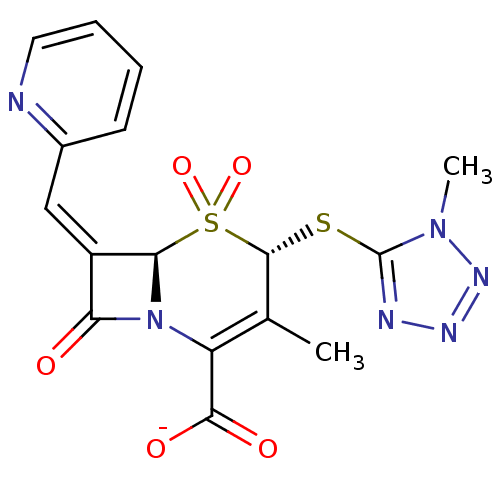 (CHEMBL355246 | Sodium; (4S,6R)-3-methyl-4-(1-methy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088812 (CHEMBL355246 | Sodium; (4S,6R)-3-methyl-4-(1-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50033701 (CHEMBL268632 | Sodium; (R)-3-acetoxymethyl-5,5,8-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088811 (CHEMBL166677 | [(R)-2-Carboxy-3-methyl-4-methylene...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50088809 (CHEMBL355648 | Sodium; (R)-7-[1-tert-butoxycarbony...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Enterobacter cloacae) | BDBM50157692 (CHEMBL1439 | CL-307579 | Sodium; (2S,3S,5R)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description Inhibition of Class C beta-lactamase from E. cloacae strain P99 | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088809 (CHEMBL355648 | Sodium; (R)-7-[1-tert-butoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088807 (CHEMBL435467 | Sodium; (R)-3,4-dimethyl-5,5,8-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.41E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088813 (CHEMBL166730 | Sodium; (R)-3-methyl-4-methylene-5,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088810 (CHEMBL353004 | Sodium; (R)-3-acetoxymethyl-4-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088807 (CHEMBL435467 | Sodium; (R)-3,4-dimethyl-5,5,8-trio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088815 (CHEMBL171001 | Sodium; (R)-4-(bis-methylsulfanyl-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088808 (CHEMBL355571 | [(R)-3-Acetoxymethyl-2-carboxy-4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088811 (CHEMBL166677 | [(R)-2-Carboxy-3-methyl-4-methylene...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50033701 (CHEMBL268632 | Sodium; (R)-3-acetoxymethyl-5,5,8-t...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088808 (CHEMBL355571 | [(R)-3-Acetoxymethyl-2-carboxy-4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.98E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088810 (CHEMBL353004 | Sodium; (R)-3-acetoxymethyl-4-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088812 (CHEMBL355246 | Sodium; (4S,6R)-3-methyl-4-(1-methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase TEM derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50033680 (CHEMBL268919 | Sodium; (R)-3-acetoxymethyl-7-[1-te...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase (Staphylococcus aureus) | BDBM50088813 (CHEMBL166730 | Sodium; (R)-3-methyl-4-methylene-5,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.86E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Methodist University Curated by ChEMBL | Assay Description The compound was evaluated for inhibition against Beta-lactamase derived from Staphylococcus aureus | Bioorg Med Chem Lett 10: 847-51 (2000) BindingDB Entry DOI: 10.7270/Q2NG4PVB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||