

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTPase HRas (Homo sapiens (Human)) | BDBM50089961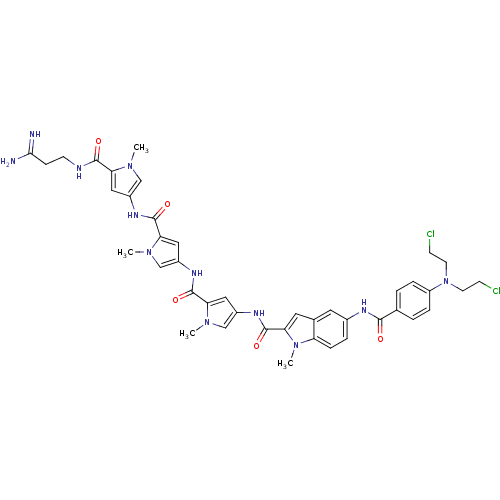 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089960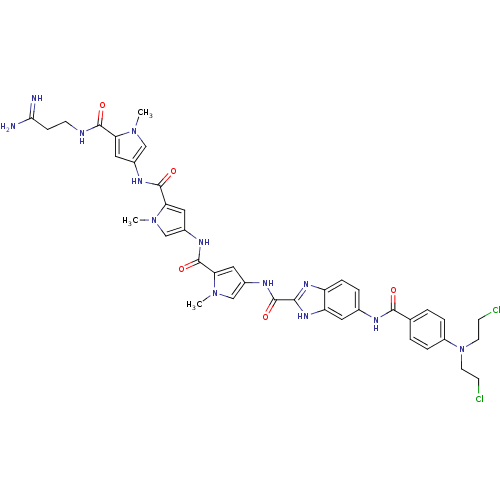 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089962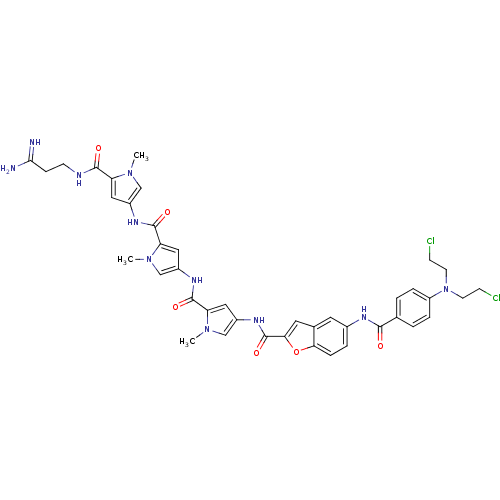 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089967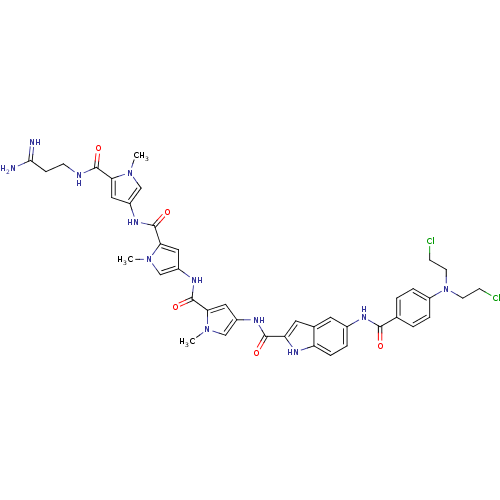 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089969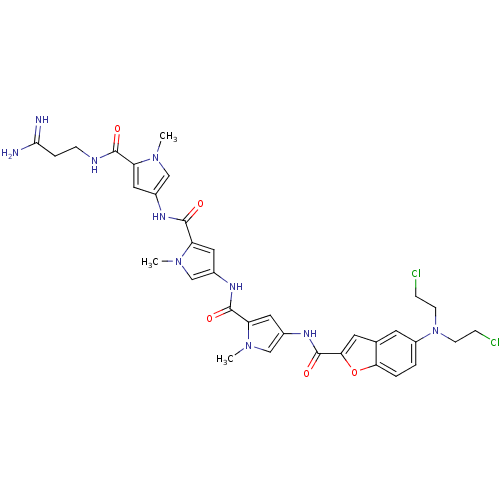 (5-[Bis-(2-chloro-ethyl)-amino]-benzofuran-2-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089971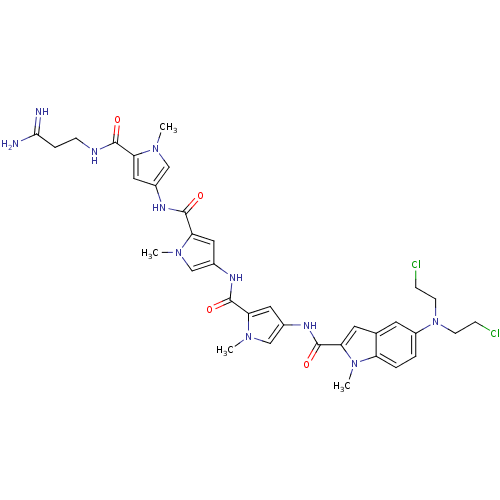 (5-[Bis-(2-chloro-ethyl)-amino]-1-methyl-1H-indole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089978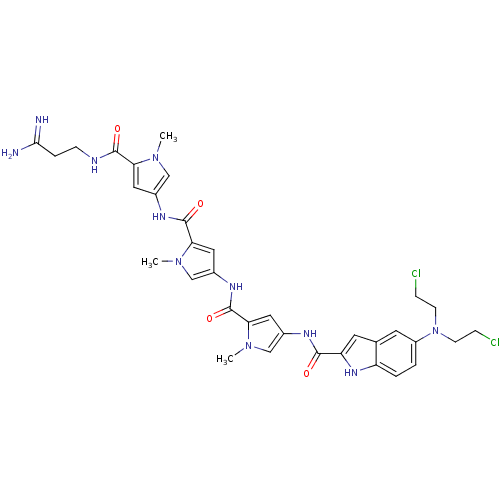 (5-[Bis-(2-chloro-ethyl)-amino]-1H-indole-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659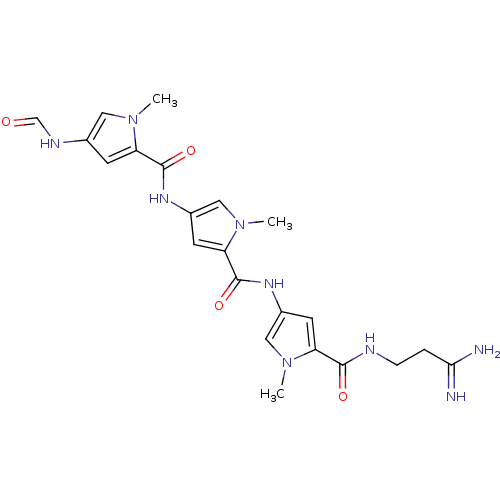 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||