

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM3085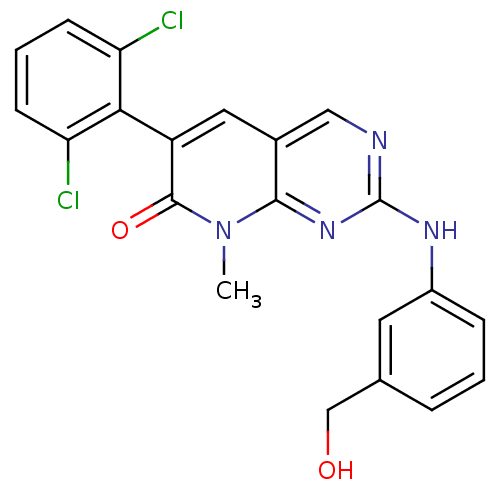 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6569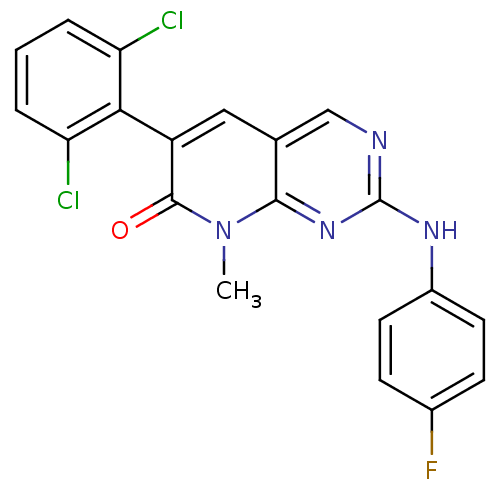 (6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6571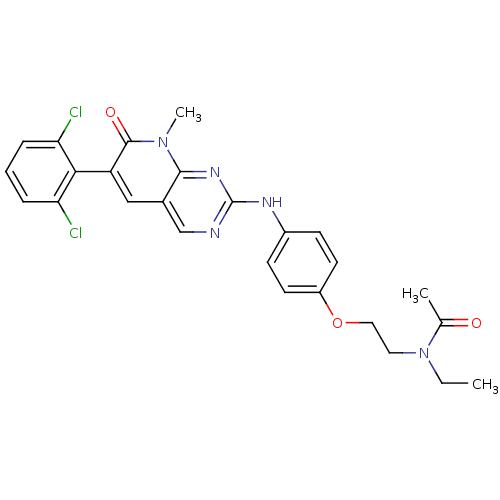 (N-[2-(4-{[6-(2,6-dichlorophenyl)-8-methyl-7-oxo-7H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM4213 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6570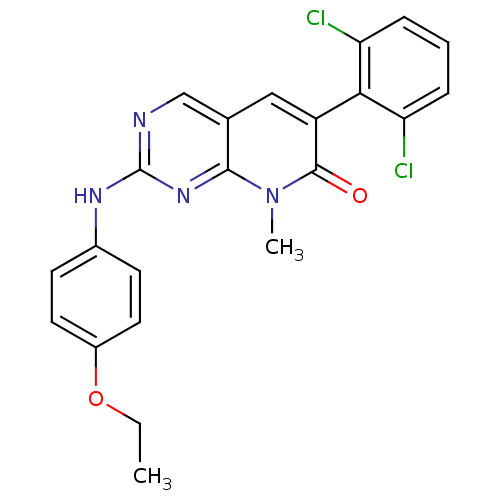 (6-(2,6-dichlorophenyl)-2-[(4-ethoxyphenyl)amino]-8...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM6572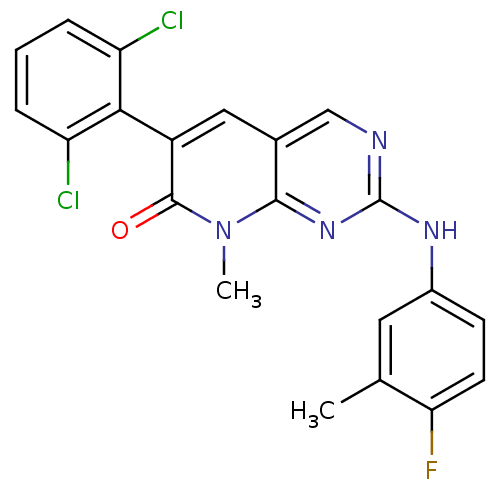 (6-(2,6-dichlorophenyl)-2-[(4-fluoro-3-methylphenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description The kinase activity was measured by DELFIA/time-resolved fluorometry. The reaction was carried out in 96-well polypropylene plates and was terminated... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM3085 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.68 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM4213 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.35 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6571 (N-[2-(4-{[6-(2,6-dichlorophenyl)-8-methyl-7-oxo-7H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.03 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6570 (6-(2,6-dichlorophenyl)-2-[(4-ethoxyphenyl)amino]-8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16.3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6572 (6-(2,6-dichlorophenyl)-2-[(4-fluoro-3-methylphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 16.8 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM3085 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 61.5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM6569 (6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 66.3 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3085 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM4213 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 98.8 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM3085 (6-(2,6-dichlorophenyl)-2-{[3-(hydroxymethyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 139 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6571 (N-[2-(4-{[6-(2,6-dichlorophenyl)-8-methyl-7-oxo-7H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 244 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM6569 (6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM6572 (6-(2,6-dichlorophenyl)-2-[(4-fluoro-3-methylphenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6570 (6-(2,6-dichlorophenyl)-2-[(4-ethoxyphenyl)amino]-8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 451 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM6571 (N-[2-(4-{[6-(2,6-dichlorophenyl)-8-methyl-7-oxo-7H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 463 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM4213 (6-(2,6-dichlorophenyl)-8-methyl-2-{[4-(morpholin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 636 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6569 (6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 933 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6572 (6-(2,6-dichlorophenyl)-2-[(4-fluoro-3-methylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 934 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM6569 (6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1 (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM6572 (6-(2,6-dichlorophenyl)-2-[(4-fluoro-3-methylphenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM6568 (6-(2,6-dichlorophenyl)-8-methyl-2-{[3-(methylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-derived growth factor receptor beta (Homo sapiens (Human)) | BDBM6570 (6-(2,6-dichlorophenyl)-2-[(4-ethoxyphenyl)amino]-8...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Parke-Davis Pharmaceutical Research | Assay Description IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe... | Biochem Pharmacol 60: 885-98 (2000) Article DOI: 10.1016/s0006-2952(00)00405-6 BindingDB Entry DOI: 10.7270/Q2MC8X7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||