

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50078263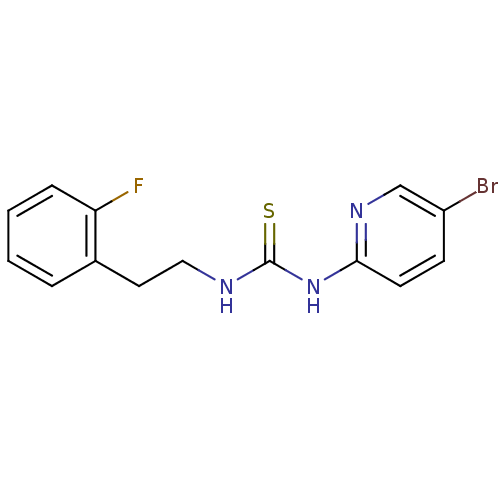 (1-(5-Bromo-pyridin-2-yl)-3-[2-(2-fluoro-phenyl)-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091963 (1-(5-Bromo-pyridin-2-yl)-3-((R)-1-cyclohexyl-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091962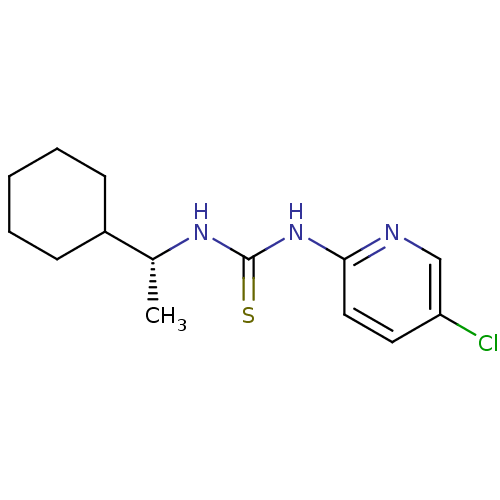 (1-(5-Chloro-pyridin-2-yl)-3-((R)-1-cyclohexyl-ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091966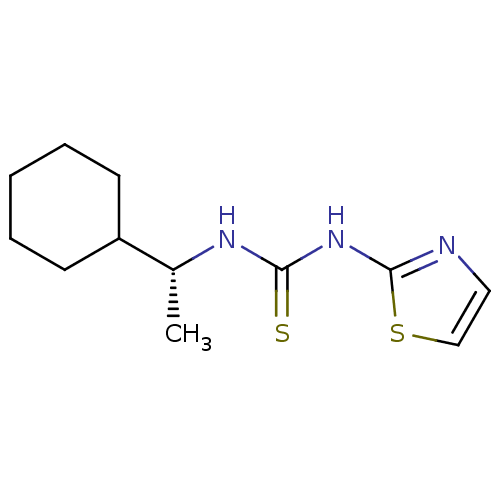 (1-((R)-1-Cyclohexyl-ethyl)-3-thiazol-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091973 (1-(5-Chloro-pyridin-2-yl)-3-((S)-1-cyclohexyl-ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091970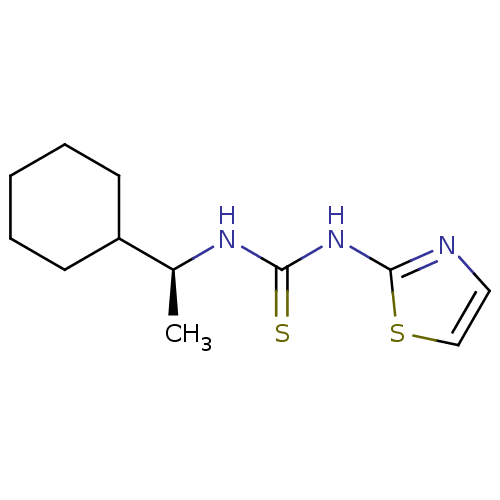 (1-((S)-1-Cyclohexyl-ethyl)-3-thiazol-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091971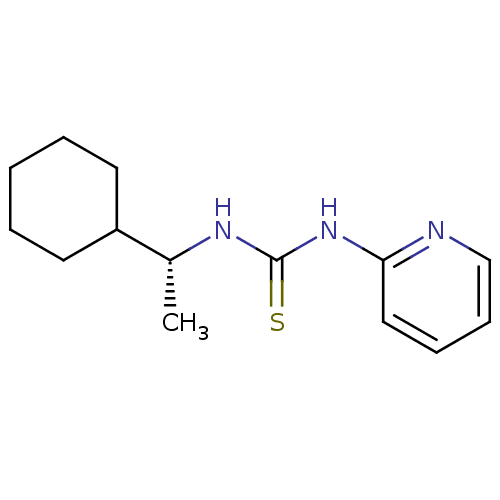 (1-((R)-1-Cyclohexyl-ethyl)-3-pyridin-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091964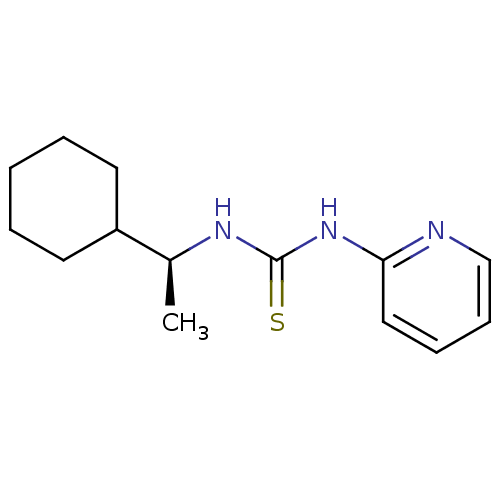 (1-((S)-1-Cyclohexyl-ethyl)-3-pyridin-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091972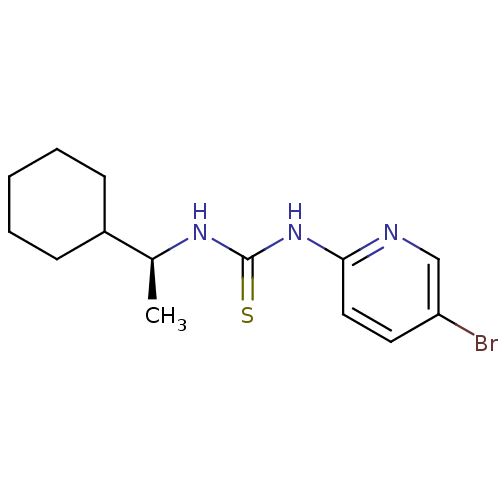 (1-(5-Bromo-pyridin-2-yl)-3-((S)-1-cyclohexyl-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50078263 (1-(5-Bromo-pyridin-2-yl)-3-[2-(2-fluoro-phenyl)-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1866 (3-(5-bromopyridin-2-yl)-1-[2-(pyridin-2-yl)ethyl]t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity against HIV reverse transcriptase (Estimated Ki) | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091963 (1-(5-Bromo-pyridin-2-yl)-3-((R)-1-cyclohexyl-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091965 (1-(5-Chloro-pyridin-2-yl)-3-((R)-1-phenyl-ethyl)-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091962 (1-(5-Chloro-pyridin-2-yl)-3-((R)-1-cyclohexyl-ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091969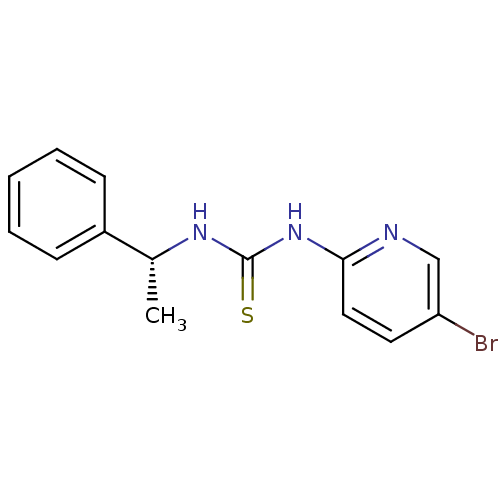 (1-(5-Bromo-pyridin-2-yl)-3-((R)-1-phenyl-ethyl)-th...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091966 (1-((R)-1-Cyclohexyl-ethyl)-3-thiazol-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091970 (1-((S)-1-Cyclohexyl-ethyl)-3-thiazol-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091964 (1-((S)-1-Cyclohexyl-ethyl)-3-pyridin-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091967 (1-(5-Chloro-pyridin-2-yl)-3-((S)-1-phenyl-ethyl)-t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091973 (1-(5-Chloro-pyridin-2-yl)-3-((S)-1-cyclohexyl-ethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091971 (1-((R)-1-Cyclohexyl-ethyl)-3-pyridin-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091968 (1-(5-Bromo-pyridin-2-yl)-3-((S)-1-phenyl-ethyl)-th...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50091972 (1-(5-Bromo-pyridin-2-yl)-3-((S)-1-cyclohexyl-ethyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against recombinant HIV-1 Reverse transcriptase using cell free RT inhibition assay | Bioorg Med Chem Lett 10: 2071-4 (2000) BindingDB Entry DOI: 10.7270/Q2668DQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||