

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacing [3H]U-69593 to human cloned Kappa opioid receptor transfected into CHO cells using [35S... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50092998 (CHEMBL72786 | Derivative of Natrindole) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor kappa 1 by displacing [3H]U-69593 | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50092999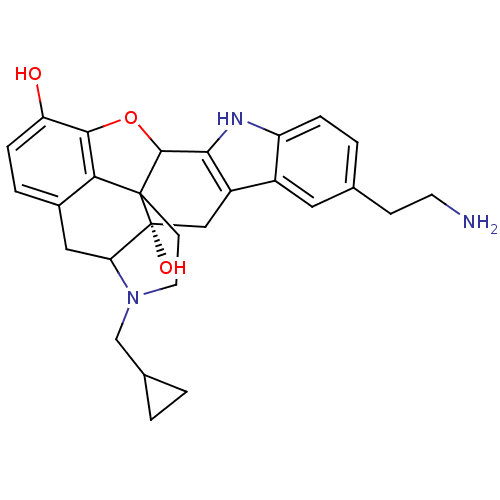 (5'-(2-aminoethyl)Natrindole | CHEMBL72885) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacing [3H]U-69593 to human cloned Kappa opioid receptor transfected into CHO cells using [35S... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093000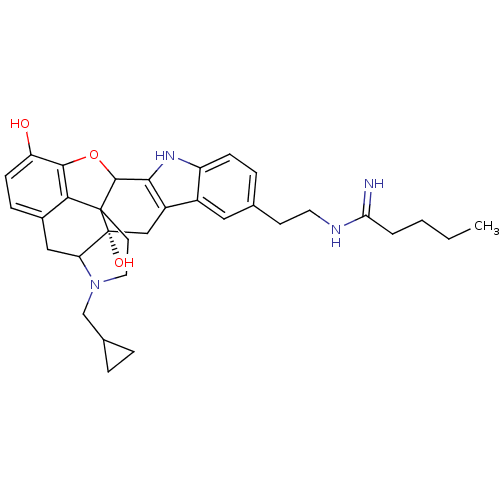 (CHEMBL70693 | Derivative of Natrindole) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacing [3H]U-69593 to human cloned Kappa opioid receptor transfected into CHO cells using [35S... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor kappa 1 by displacing [3H]U-69593 | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50093000 (CHEMBL70693 | Derivative of Natrindole) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor kappa 1 by displacing [3H]U-69593 | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50092999 (5'-(2-aminoethyl)Natrindole | CHEMBL72885) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor kappa 1 by displacing [3H]U-69593 | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50093000 (CHEMBL70693 | Derivative of Natrindole) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for functional opioid activity by stimulation of [35S]GTP-gamma-S, in cloned human Opioid receptor delta 1 transfected into C... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50092998 (CHEMBL72786 | Derivative of Natrindole) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against Opioid receptor mu 1 of guinea pig brain membrane by displacing [3H]DAMGO | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50092999 (5'-(2-aminoethyl)Natrindole | CHEMBL72885) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for functional opioid activity by stimulation of [35S]GTP-gamma-S, in cloned human Opioid receptor delta 1 transfected into C... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for functional opioid activity by stimulation of [35S]GTP-gamma-S, in cloned human Opioid receptor delta 1 transfected into C... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50093000 (CHEMBL70693 | Derivative of Natrindole) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacing DAMGO to human cloned mu opioid receptor transfected into CHO cells using [35S]GTP-gamm... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50092998 (CHEMBL72786 | Derivative of Natrindole) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor delta 1 by displacement of [3H]-Cl-DPDPE | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor delta 1 by displacement of [3H]Cl-DPDPE | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacing DAMGO to human cloned mu opioid receptor transfected into CHO cells using [35S]GTP-gamm... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor mu 1 by displacing [3H]-DAMGO | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50092999 (5'-(2-aminoethyl)Natrindole | CHEMBL72885) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor delta 1 by displacement of [3H]Cl-DPDPE | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50092999 (5'-(2-aminoethyl)Natrindole | CHEMBL72885) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against CHO cells transfected with cloned human Opioid receptor mu 1 by displacing [3H]DAMGO | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50092999 (5'-(2-aminoethyl)Natrindole | CHEMBL72885) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity to CHO cells expressing cloned human Opioid receptor delta 1 by displacement of [3H]Cl-DPDPE | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50093000 (CHEMBL70693 | Derivative of Natrindole) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity against CHO cells transfected with cloned human Opioid receptor mu 1 by displacing [3H]DAMGO | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50093000 (CHEMBL70693 | Derivative of Natrindole) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 219 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bristol Curated by ChEMBL | Assay Description Compound was evaluated for its binding affinity by displacing [3H]U-69593 to human cloned Kappa opioid receptor transfected into CHO cells using [35S... | Bioorg Med Chem Lett 10: 2259-61 (2001) BindingDB Entry DOI: 10.7270/Q2WS8TRF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||