 Found 72 hits of Enzyme Inhibition Constant Data
Found 72 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50084616
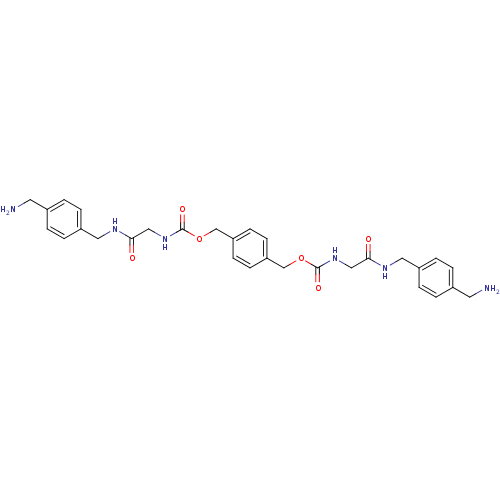
(CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...)Show SMILES NCc1ccc(CNC(=O)CNC(=O)OCc2ccc(COC(=O)NCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O6/c31-13-21-1-5-23(6-2-21)15-33-27(37)17-35-29(39)41-19-25-9-11-26(12-10-25)20-42-30(40)36-18-28(38)34-16-24-7-3-22(14-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093132
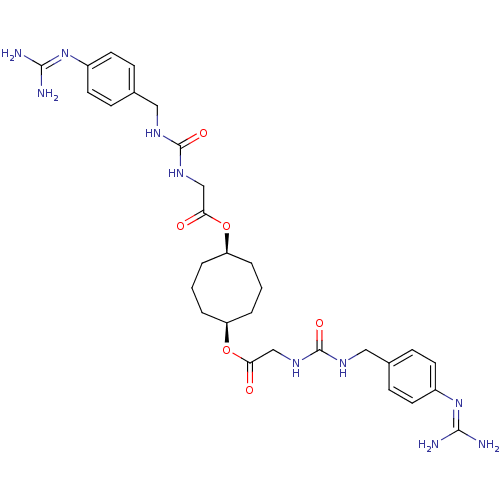
(CHEMBL75749 | [3-(4-Guanidino-benzyl)-ureido]-acet...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H42N10O6/c31-27(32)39-21-11-7-19(8-12-21)15-35-29(43)37-17-25(41)45-23-3-1-4-24(6-2-5-23)46-26(42)18-38-30(44)36-16-20-9-13-22(14-10-20)40-28(33)34/h7-14,23-24H,1-6,15-18H2,(H4,31,32,39)(H4,33,34,40)(H2,35,37,43)(H2,36,38,44)/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093143
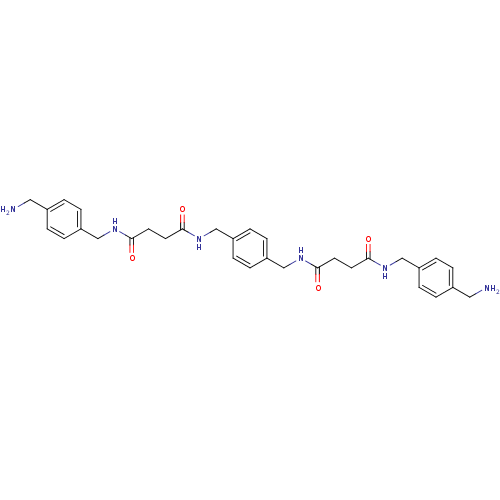
(CHEMBL76733 | N-(4-Aminomethyl-benzyl)-N'-(4-{[3-(...)Show SMILES NCc1ccc(CNC(=O)CCC(=O)NCc2ccc(CNC(=O)CCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C32H40N6O4/c33-17-23-1-5-25(6-2-23)19-35-29(39)13-15-31(41)37-21-27-9-11-28(12-10-27)22-38-32(42)16-14-30(40)36-20-26-7-3-24(18-34)4-8-26/h1-12H,13-22,33-34H2,(H,35,39)(H,36,40)(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093133
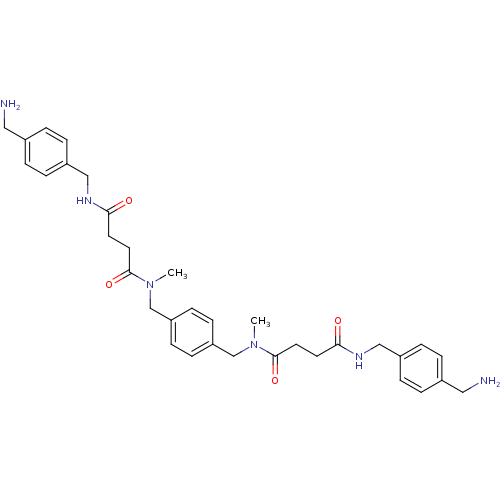
(CHEMBL79822 | N-(4-Aminomethyl-benzyl)-N'-[4-({[3-...)Show SMILES CN(Cc1ccc(CN(C)C(=O)CCC(=O)NCc2ccc(CN)cc2)cc1)C(=O)CCC(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(33(43)17-15-31(41)37-21-27-7-3-25(19-35)4-8-27)23-29-11-13-30(14-12-29)24-40(2)34(44)18-16-32(42)38-22-28-9-5-26(20-36)6-10-28/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093131
(CHEMBL80210 | [(4-Aminomethyl-benzylcarbamoyl)-met...)Show SMILES CN(CC(=O)NCc1ccc(CN)cc1)C(=O)OCc1ccc(COC(=O)N(C)CC(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C32H40N6O6/c1-37(19-29(39)35-17-25-7-3-23(15-33)4-8-25)31(41)43-21-27-11-13-28(14-12-27)22-44-32(42)38(2)20-30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14H,15-22,33-34H2,1-2H3,(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093141
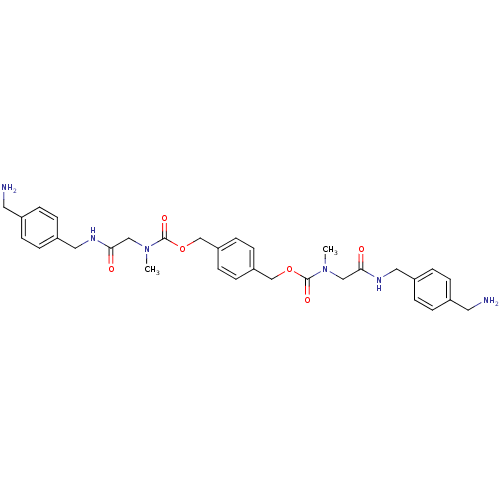
(CHEMBL306537 | Pentanedioic acid 4-aminomethyl-ben...)Show SMILES NCc1ccc(CNC(=O)CCCC(=O)NCc2ccc(CNC(=O)CCCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c35-19-25-7-11-27(12-8-25)21-37-31(41)3-1-5-33(43)39-23-29-15-17-30(18-16-29)24-40-34(44)6-2-4-32(42)38-22-28-13-9-26(20-36)10-14-28/h7-18H,1-6,19-24,35-36H2,(H,37,41)(H,38,42)(H,39,43)(H,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093135
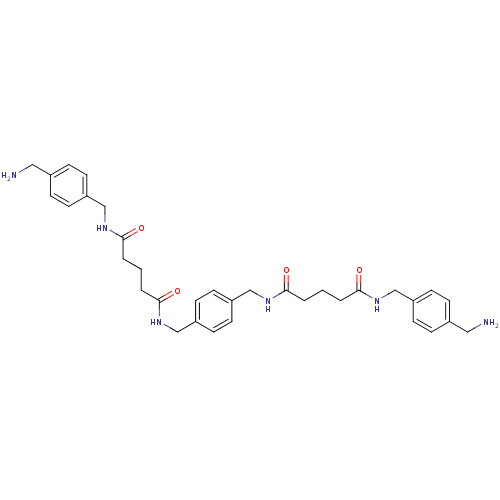
(CHEMBL77911 | [(4-Guanidino-benzylcarbamoyl)-methy...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O6/c31-27(32)39-23-9-5-19(6-10-23)13-35-25(41)15-37-29(43)45-17-21-1-2-22(4-3-21)18-46-30(44)38-16-26(42)36-14-20-7-11-24(12-8-20)40-28(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093139
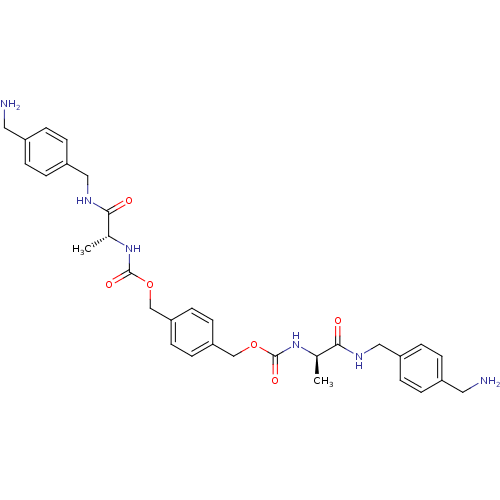
(CHEMBL310289 | [(R)-1-(4-Aminomethyl-benzylcarbamo...)Show SMILES C[C@@H](NC(=O)OCc1ccc(COC(=O)N[C@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093136
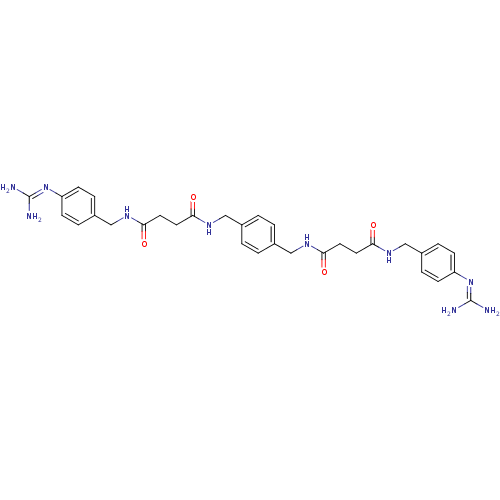
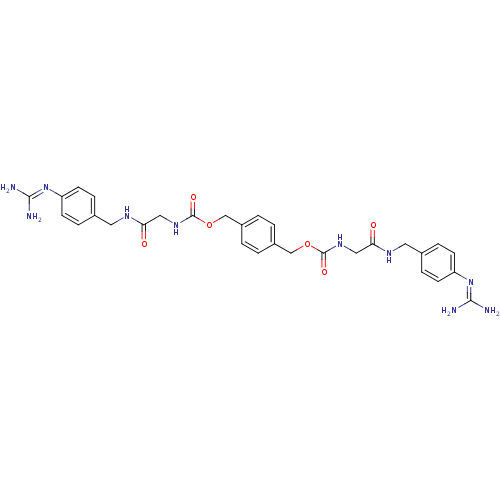
(CHEMBL309788 | N-(4-Guanidino-benzyl)-N'-(4-{[3-(4...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O4/c33-31(34)41-25-9-5-23(6-10-25)19-39-29(45)15-13-27(43)37-17-21-1-2-22(4-3-21)18-38-28(44)14-16-30(46)40-20-24-7-11-26(12-8-24)42-32(35)36/h1-12H,13-20H2,(H,37,43)(H,38,44)(H,39,45)(H,40,46)(H4,33,34,41)(H4,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093145
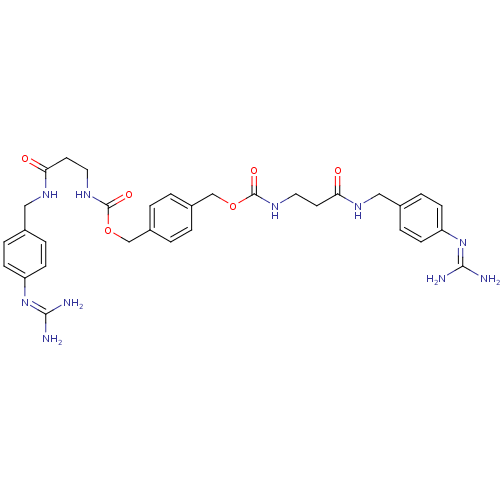
(CHEMBL408300 | [2-(4-Guanidino-benzylcarbamoyl)-et...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O6/c33-29(34)41-25-9-5-21(6-10-25)17-39-27(43)13-15-37-31(45)47-19-23-1-2-24(4-3-23)20-48-32(46)38-16-14-28(44)40-18-22-7-11-26(12-8-22)42-30(35)36/h1-12H,13-20H2,(H,37,45)(H,38,46)(H,39,43)(H,40,44)(H4,33,34,41)(H4,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093147
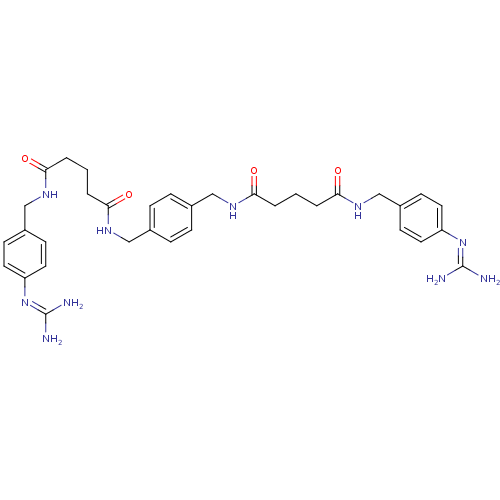
(CHEMBL79545 | Pentanedioic acid 4-guanidino-benzyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C34H44N10O4/c35-33(36)43-27-15-11-25(12-16-27)21-41-31(47)5-1-3-29(45)39-19-23-7-9-24(10-8-23)20-40-30(46)4-2-6-32(48)42-22-26-13-17-28(18-14-26)44-34(37)38/h7-18H,1-6,19-22H2,(H,39,45)(H,40,46)(H,41,47)(H,42,48)(H4,35,36,43)(H4,37,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093146
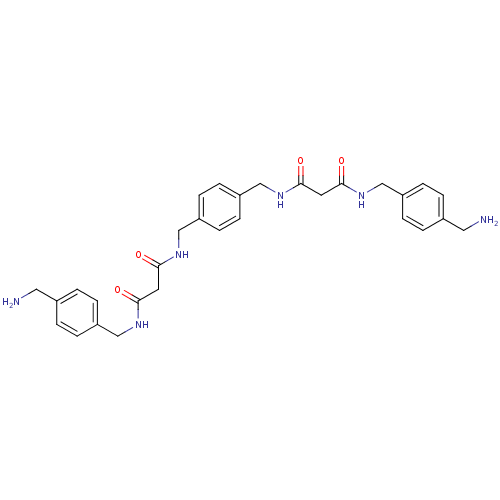
(CHEMBL77521 | N-(4-Aminomethyl-benzyl)-N'-(4-{[2-(...)Show SMILES NCc1ccc(CNC(=O)CC(=O)NCc2ccc(CNC(=O)CC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O4/c31-15-21-1-5-23(6-2-21)17-33-27(37)13-29(39)35-19-25-9-11-26(12-10-25)20-36-30(40)14-28(38)34-18-24-7-3-22(16-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093137
(CHEMBL80149 | [(S)-1-(4-Aminomethyl-benzylcarbamoy...)Show SMILES C[C@H](NC(=O)OCc1ccc(COC(=O)N[C@@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 366 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093144
(CHEMBL46120 | N-(4-Guanidino-benzyl)-N'-(4-{[2-(4-...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](\[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O4/c31-29(32)39-23-9-5-21(6-10-23)17-37-27(43)13-25(41)35-15-19-1-2-20(4-3-19)16-36-26(42)14-28(44)38-18-22-7-11-24(12-8-22)40-30(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093138
(CHEMBL78757 | N-(4-Aminomethyl-benzyl)-N'-[4-({3-[...)Show SMILES CN(Cc1ccc(CN)cc1)C(=O)CCC(=O)NCc1ccc(CNC(=O)CCC(=O)N(C)Cc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(23-29-11-3-25(19-35)4-12-29)33(43)17-15-31(41)37-21-27-7-9-28(10-8-27)22-38-32(42)16-18-34(44)40(2)24-30-13-5-26(20-36)6-14-30/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093140
(CHEMBL43463 | Derivative of piperazine-1-carboxyli...)Show SMILES O=C(NCc1ccccc1)N1CCN(CC1)C(=O)O[C@H]1CCC[C@H](CCC1)OC(=O)N1CCN(CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C34H46N6O6/c41-31(35-25-27-9-3-1-4-10-27)37-17-21-39(22-18-37)33(43)45-29-13-7-15-30(16-8-14-29)46-34(44)40-23-19-38(20-24-40)32(42)36-26-28-11-5-2-6-12-28/h1-6,9-12,29-30H,7-8,13-26H2,(H,35,41)(H,36,42)/t29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093134
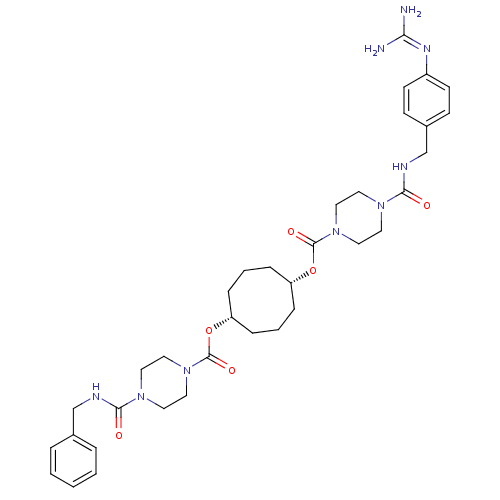
(CHEMBL79655 | Derivative of piperazine-1-carboxyli...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@@H]-2-[#6]-[#6]-[#6]-[#6@@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C35H49N9O6/c36-31(37)40-28-14-12-27(13-15-28)25-39-33(46)42-18-22-44(23-19-42)35(48)50-30-10-4-8-29(9-5-11-30)49-34(47)43-20-16-41(17-21-43)32(45)38-24-26-6-2-1-3-7-26/h1-3,6-7,12-15,29-30H,4-5,8-11,16-25H2,(H,38,45)(H,39,46)(H4,36,37,40)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093139

(CHEMBL310289 | [(R)-1-(4-Aminomethyl-benzylcarbamo...)Show SMILES C[C@@H](NC(=O)OCc1ccc(COC(=O)N[C@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093142
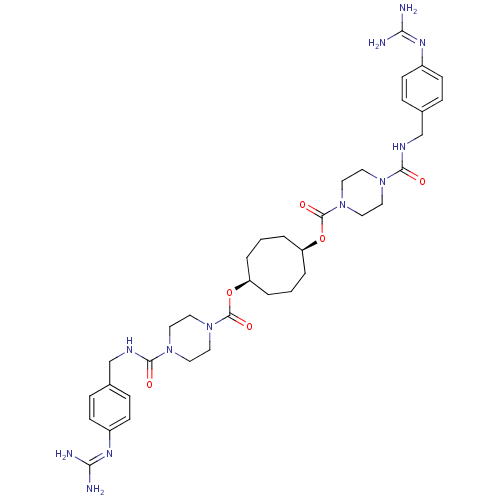
(1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093140

(CHEMBL43463 | Derivative of piperazine-1-carboxyli...)Show SMILES O=C(NCc1ccccc1)N1CCN(CC1)C(=O)O[C@H]1CCC[C@H](CCC1)OC(=O)N1CCN(CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C34H46N6O6/c41-31(35-25-27-9-3-1-4-10-27)37-17-21-39(22-18-37)33(43)45-29-13-7-15-30(16-8-14-29)46-34(44)40-23-19-38(20-24-40)32(42)36-26-28-11-5-2-6-12-28/h1-6,9-12,29-30H,7-8,13-26H2,(H,35,41)(H,36,42)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093131

(CHEMBL80210 | [(4-Aminomethyl-benzylcarbamoyl)-met...)Show SMILES CN(CC(=O)NCc1ccc(CN)cc1)C(=O)OCc1ccc(COC(=O)N(C)CC(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C32H40N6O6/c1-37(19-29(39)35-17-25-7-3-23(15-33)4-8-25)31(41)43-21-27-11-13-28(14-12-27)22-44-32(42)38(2)20-30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14H,15-22,33-34H2,1-2H3,(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093135

(CHEMBL77911 | [(4-Guanidino-benzylcarbamoyl)-methy...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O6/c31-27(32)39-23-9-5-19(6-10-23)13-35-25(41)15-37-29(43)45-17-21-1-2-22(4-3-21)18-46-30(44)38-16-26(42)36-14-20-7-11-24(12-8-20)40-28(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093147

(CHEMBL79545 | Pentanedioic acid 4-guanidino-benzyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C34H44N10O4/c35-33(36)43-27-15-11-25(12-16-27)21-41-31(47)5-1-3-29(45)39-19-23-7-9-24(10-8-23)20-40-30(46)4-2-6-32(48)42-22-26-13-17-28(18-14-26)44-34(37)38/h7-18H,1-6,19-22H2,(H,39,45)(H,40,46)(H,41,47)(H,42,48)(H4,35,36,43)(H4,37,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093145

(CHEMBL408300 | [2-(4-Guanidino-benzylcarbamoyl)-et...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O6/c33-29(34)41-25-9-5-21(6-10-25)17-39-27(43)13-15-37-31(45)47-19-23-1-2-24(4-3-23)20-48-32(46)38-16-14-28(44)40-18-22-7-11-26(12-8-22)42-30(35)36/h1-12H,13-20H2,(H,37,45)(H,38,46)(H,39,43)(H,40,44)(H4,33,34,41)(H4,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093144

(CHEMBL46120 | N-(4-Guanidino-benzyl)-N'-(4-{[2-(4-...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](\[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O4/c31-29(32)39-23-9-5-21(6-10-23)17-37-27(43)13-25(41)35-15-19-1-2-20(4-3-19)16-36-26(42)14-28(44)38-18-22-7-11-24(12-8-22)40-30(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093132

(CHEMBL75749 | [3-(4-Guanidino-benzyl)-ureido]-acet...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H42N10O6/c31-27(32)39-21-11-7-19(8-12-21)15-35-29(43)37-17-25(41)45-23-3-1-4-24(6-2-5-23)46-26(42)18-38-30(44)36-16-20-9-13-22(14-10-20)40-28(33)34/h7-14,23-24H,1-6,15-18H2,(H4,31,32,39)(H4,33,34,40)(H2,35,37,43)(H2,36,38,44)/t23-,24+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093137

(CHEMBL80149 | [(S)-1-(4-Aminomethyl-benzylcarbamoy...)Show SMILES C[C@H](NC(=O)OCc1ccc(COC(=O)N[C@@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50084616

(CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...)Show SMILES NCc1ccc(CNC(=O)CNC(=O)OCc2ccc(COC(=O)NCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O6/c31-13-21-1-5-23(6-2-21)15-33-27(37)17-35-29(39)41-19-25-9-11-26(12-10-25)20-42-30(40)36-18-28(38)34-16-24-7-3-22(14-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase beta-2
(Homo sapiens (Human)) | BDBM50093140

(CHEMBL43463 | Derivative of piperazine-1-carboxyli...)Show SMILES O=C(NCc1ccccc1)N1CCN(CC1)C(=O)O[C@H]1CCC[C@H](CCC1)OC(=O)N1CCN(CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C34H46N6O6/c41-31(35-25-27-9-3-1-4-10-27)37-17-21-39(22-18-37)33(43)45-29-13-7-15-30(16-8-14-29)46-34(44)40-23-19-38(20-24-40)32(42)36-26-28-11-5-2-6-12-28/h1-6,9-12,29-30H,7-8,13-26H2,(H,35,41)(H,36,42)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against tryptase |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093136

(CHEMBL309788 | N-(4-Guanidino-benzyl)-N'-(4-{[3-(4...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O4/c33-31(34)41-25-9-5-23(6-10-25)19-39-29(45)15-13-27(43)37-17-21-1-2-22(4-3-21)18-38-28(44)14-16-30(46)40-20-24-7-11-26(12-8-24)42-32(35)36/h1-12H,13-20H2,(H,37,43)(H,38,44)(H,39,45)(H,40,46)(H4,33,34,41)(H4,35,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093133

(CHEMBL79822 | N-(4-Aminomethyl-benzyl)-N'-[4-({[3-...)Show SMILES CN(Cc1ccc(CN(C)C(=O)CCC(=O)NCc2ccc(CN)cc2)cc1)C(=O)CCC(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(33(43)17-15-31(41)37-21-27-7-3-25(19-35)4-8-27)23-29-11-13-30(14-12-29)24-40(2)34(44)18-16-32(42)38-22-28-9-5-26(20-36)6-10-28/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093134

(CHEMBL79655 | Derivative of piperazine-1-carboxyli...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@@H]-2-[#6]-[#6]-[#6]-[#6@@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C35H49N9O6/c36-31(37)40-28-14-12-27(13-15-28)25-39-33(46)42-18-22-44(23-19-42)35(48)50-30-10-4-8-29(9-5-11-30)49-34(47)43-20-16-41(17-21-43)32(45)38-24-26-6-2-1-3-7-26/h1-3,6-7,12-15,29-30H,4-5,8-11,16-25H2,(H,38,45)(H,39,46)(H4,36,37,40)/t29-,30+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093133

(CHEMBL79822 | N-(4-Aminomethyl-benzyl)-N'-[4-({[3-...)Show SMILES CN(Cc1ccc(CN(C)C(=O)CCC(=O)NCc2ccc(CN)cc2)cc1)C(=O)CCC(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(33(43)17-15-31(41)37-21-27-7-3-25(19-35)4-8-27)23-29-11-13-30(14-12-29)24-40(2)34(44)18-16-32(42)38-22-28-9-5-26(20-36)6-10-28/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093131

(CHEMBL80210 | [(4-Aminomethyl-benzylcarbamoyl)-met...)Show SMILES CN(CC(=O)NCc1ccc(CN)cc1)C(=O)OCc1ccc(COC(=O)N(C)CC(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C32H40N6O6/c1-37(19-29(39)35-17-25-7-3-23(15-33)4-8-25)31(41)43-21-27-11-13-28(14-12-27)22-44-32(42)38(2)20-30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14H,15-22,33-34H2,1-2H3,(H,35,39)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093143

(CHEMBL76733 | N-(4-Aminomethyl-benzyl)-N'-(4-{[3-(...)Show SMILES NCc1ccc(CNC(=O)CCC(=O)NCc2ccc(CNC(=O)CCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C32H40N6O4/c33-17-23-1-5-25(6-2-23)19-35-29(39)13-15-31(41)37-21-27-9-11-28(12-10-27)22-38-32(42)16-14-30(40)36-20-26-7-3-24(18-34)4-8-26/h1-12H,13-22,33-34H2,(H,35,39)(H,36,40)(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093137

(CHEMBL80149 | [(S)-1-(4-Aminomethyl-benzylcarbamoy...)Show SMILES C[C@H](NC(=O)OCc1ccc(COC(=O)N[C@@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.73E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093141

(CHEMBL306537 | Pentanedioic acid 4-aminomethyl-ben...)Show SMILES NCc1ccc(CNC(=O)CCCC(=O)NCc2ccc(CNC(=O)CCCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c35-19-25-7-11-27(12-8-25)21-37-31(41)3-1-5-33(43)39-23-29-15-17-30(18-16-29)24-40-34(44)6-2-4-32(42)38-22-28-13-9-26(20-36)10-14-28/h7-18H,1-6,19-24,35-36H2,(H,37,41)(H,38,42)(H,39,43)(H,40,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.74E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093146

(CHEMBL77521 | N-(4-Aminomethyl-benzyl)-N'-(4-{[2-(...)Show SMILES NCc1ccc(CNC(=O)CC(=O)NCc2ccc(CNC(=O)CC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O4/c31-15-21-1-5-23(6-2-21)17-33-27(37)13-29(39)35-19-25-9-11-26(12-10-25)20-36-30(40)14-28(38)34-18-24-7-3-22(16-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 3.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093142

(1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093147

(CHEMBL79545 | Pentanedioic acid 4-guanidino-benzyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C34H44N10O4/c35-33(36)43-27-15-11-25(12-16-27)21-41-31(47)5-1-3-29(45)39-19-23-7-9-24(10-8-23)20-40-30(46)4-2-6-32(48)42-22-26-13-17-28(18-14-26)44-34(37)38/h7-18H,1-6,19-22H2,(H,39,45)(H,40,46)(H,41,47)(H,42,48)(H4,35,36,43)(H4,37,38,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.92E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093142

(1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 4.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093143

(CHEMBL76733 | N-(4-Aminomethyl-benzyl)-N'-(4-{[3-(...)Show SMILES NCc1ccc(CNC(=O)CCC(=O)NCc2ccc(CNC(=O)CCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C32H40N6O4/c33-17-23-1-5-25(6-2-23)19-35-29(39)13-15-31(41)37-21-27-9-11-28(12-10-27)22-38-32(42)16-14-30(40)36-20-26-7-3-24(18-34)4-8-26/h1-12H,13-22,33-34H2,(H,35,39)(H,36,40)(H,37,41)(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093135

(CHEMBL77911 | [(4-Guanidino-benzylcarbamoyl)-methy...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O6/c31-27(32)39-23-9-5-19(6-10-23)13-35-25(41)15-37-29(43)45-17-21-1-2-22(4-3-21)18-46-30(44)38-16-26(42)36-14-20-7-11-24(12-8-20)40-28(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093138

(CHEMBL78757 | N-(4-Aminomethyl-benzyl)-N'-[4-({3-[...)Show SMILES CN(Cc1ccc(CN)cc1)C(=O)CCC(=O)NCc1ccc(CNC(=O)CCC(=O)N(C)Cc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(23-29-11-3-25(19-35)4-12-29)33(43)17-15-31(41)37-21-27-7-9-28(10-8-27)22-38-32(42)16-18-34(44)40(2)24-30-13-5-26(20-36)6-14-30/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093143

(CHEMBL76733 | N-(4-Aminomethyl-benzyl)-N'-(4-{[3-(...)Show SMILES NCc1ccc(CNC(=O)CCC(=O)NCc2ccc(CNC(=O)CCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C32H40N6O4/c33-17-23-1-5-25(6-2-23)19-35-29(39)13-15-31(41)37-21-27-9-11-28(12-10-27)22-38-32(42)16-14-30(40)36-20-26-7-3-24(18-34)4-8-26/h1-12H,13-22,33-34H2,(H,35,39)(H,36,40)(H,37,41)(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093145

(CHEMBL408300 | [2-(4-Guanidino-benzylcarbamoyl)-et...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O6/c33-29(34)41-25-9-5-21(6-10-25)17-39-27(43)13-15-37-31(45)47-19-23-1-2-24(4-3-23)20-48-32(46)38-16-14-28(44)40-18-22-7-11-26(12-8-22)42-30(35)36/h1-12H,13-20H2,(H,37,45)(H,38,46)(H,39,43)(H,40,44)(H4,33,34,41)(H4,35,36,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093132

(CHEMBL75749 | [3-(4-Guanidino-benzyl)-ureido]-acet...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H42N10O6/c31-27(32)39-21-11-7-19(8-12-21)15-35-29(43)37-17-25(41)45-23-3-1-4-24(6-2-5-23)46-26(42)18-38-30(44)36-16-20-9-13-22(14-10-20)40-28(33)34/h7-14,23-24H,1-6,15-18H2,(H4,31,32,39)(H4,33,34,40)(H2,35,37,43)(H2,36,38,44)/t23-,24+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093141

(CHEMBL306537 | Pentanedioic acid 4-aminomethyl-ben...)Show SMILES NCc1ccc(CNC(=O)CCCC(=O)NCc2ccc(CNC(=O)CCCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c35-19-25-7-11-27(12-8-25)21-37-31(41)3-1-5-33(43)39-23-29-15-17-30(18-16-29)24-40-34(44)6-2-4-32(42)38-22-28-13-9-26(20-36)10-14-28/h7-18H,1-6,19-24,35-36H2,(H,37,41)(H,38,42)(H,39,43)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093147

(CHEMBL79545 | Pentanedioic acid 4-guanidino-benzyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C34H44N10O4/c35-33(36)43-27-15-11-25(12-16-27)21-41-31(47)5-1-3-29(45)39-19-23-7-9-24(10-8-23)20-40-30(46)4-2-6-32(48)42-22-26-13-17-28(18-14-26)44-34(37)38/h7-18H,1-6,19-22H2,(H,39,45)(H,40,46)(H,41,47)(H,42,48)(H4,35,36,43)(H4,37,38,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093139

(CHEMBL310289 | [(R)-1-(4-Aminomethyl-benzylcarbamo...)Show SMILES C[C@@H](NC(=O)OCc1ccc(COC(=O)N[C@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093144

(CHEMBL46120 | N-(4-Guanidino-benzyl)-N'-(4-{[2-(4-...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](\[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O4/c31-29(32)39-23-9-5-21(6-10-23)17-37-27(43)13-25(41)35-15-19-1-2-20(4-3-19)16-36-26(42)14-28(44)38-18-22-7-11-24(12-8-22)40-30(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093146

(CHEMBL77521 | N-(4-Aminomethyl-benzyl)-N'-(4-{[2-(...)Show SMILES NCc1ccc(CNC(=O)CC(=O)NCc2ccc(CNC(=O)CC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O4/c31-15-21-1-5-23(6-2-21)17-33-27(37)13-29(39)35-19-25-9-11-26(12-10-25)20-36-30(40)14-28(38)34-18-24-7-3-22(16-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093133

(CHEMBL79822 | N-(4-Aminomethyl-benzyl)-N'-[4-({[3-...)Show SMILES CN(Cc1ccc(CN(C)C(=O)CCC(=O)NCc2ccc(CN)cc2)cc1)C(=O)CCC(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(33(43)17-15-31(41)37-21-27-7-3-25(19-35)4-8-27)23-29-11-13-30(14-12-29)24-40(2)34(44)18-16-32(42)38-22-28-9-5-26(20-36)6-10-28/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50093138

(CHEMBL78757 | N-(4-Aminomethyl-benzyl)-N'-[4-({3-[...)Show SMILES CN(Cc1ccc(CN)cc1)C(=O)CCC(=O)NCc1ccc(CNC(=O)CCC(=O)N(C)Cc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(23-29-11-3-25(19-35)4-12-29)33(43)17-15-31(41)37-21-27-7-9-28(10-8-27)22-38-32(42)16-18-34(44)40(2)24-30-13-5-26(20-36)6-14-30/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against trypsin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093134

(CHEMBL79655 | Derivative of piperazine-1-carboxyli...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@@H]-2-[#6]-[#6]-[#6]-[#6@@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C35H49N9O6/c36-31(37)40-28-14-12-27(13-15-28)25-39-33(46)42-18-22-44(23-19-42)35(48)50-30-10-4-8-29(9-5-11-30)49-34(47)43-20-16-41(17-21-43)32(45)38-24-26-6-2-1-3-7-26/h1-3,6-7,12-15,29-30H,4-5,8-11,16-25H2,(H,38,45)(H,39,46)(H4,36,37,40)/t29-,30+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093138

(CHEMBL78757 | N-(4-Aminomethyl-benzyl)-N'-[4-({3-[...)Show SMILES CN(Cc1ccc(CN)cc1)C(=O)CCC(=O)NCc1ccc(CNC(=O)CCC(=O)N(C)Cc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c1-39(23-29-11-3-25(19-35)4-12-29)33(43)17-15-31(41)37-21-27-7-9-28(10-8-27)22-38-32(42)16-18-34(44)40(2)24-30-13-5-26(20-36)6-14-30/h3-14H,15-24,35-36H2,1-2H3,(H,37,41)(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093139

(CHEMBL310289 | [(R)-1-(4-Aminomethyl-benzylcarbamo...)Show SMILES C[C@@H](NC(=O)OCc1ccc(COC(=O)N[C@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093134

(CHEMBL79655 | Derivative of piperazine-1-carboxyli...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@@H]-2-[#6]-[#6]-[#6]-[#6@@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccccc2)cc1 Show InChI InChI=1S/C35H49N9O6/c36-31(37)40-28-14-12-27(13-15-28)25-39-33(46)42-18-22-44(23-19-42)35(48)50-30-10-4-8-29(9-5-11-30)49-34(47)43-20-16-41(17-21-43)32(45)38-24-26-6-2-1-3-7-26/h1-3,6-7,12-15,29-30H,4-5,8-11,16-25H2,(H,38,45)(H,39,46)(H4,36,37,40)/t29-,30+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093131

(CHEMBL80210 | [(4-Aminomethyl-benzylcarbamoyl)-met...)Show SMILES CN(CC(=O)NCc1ccc(CN)cc1)C(=O)OCc1ccc(COC(=O)N(C)CC(=O)NCc2ccc(CN)cc2)cc1 Show InChI InChI=1S/C32H40N6O6/c1-37(19-29(39)35-17-25-7-3-23(15-33)4-8-25)31(41)43-21-27-11-13-28(14-12-27)22-44-32(42)38(2)20-30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14H,15-22,33-34H2,1-2H3,(H,35,39)(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093135

(CHEMBL77911 | [(4-Guanidino-benzylcarbamoyl)-methy...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O6/c31-27(32)39-23-9-5-19(6-10-23)13-35-25(41)15-37-29(43)45-17-21-1-2-22(4-3-21)18-46-30(44)38-16-26(42)36-14-20-7-11-24(12-8-20)40-28(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093146

(CHEMBL77521 | N-(4-Aminomethyl-benzyl)-N'-(4-{[2-(...)Show SMILES NCc1ccc(CNC(=O)CC(=O)NCc2ccc(CNC(=O)CC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O4/c31-15-21-1-5-23(6-2-21)17-33-27(37)13-29(39)35-19-25-9-11-26(12-10-25)20-36-30(40)14-28(38)34-18-24-7-3-22(16-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093141

(CHEMBL306537 | Pentanedioic acid 4-aminomethyl-ben...)Show SMILES NCc1ccc(CNC(=O)CCCC(=O)NCc2ccc(CNC(=O)CCCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C34H44N6O4/c35-19-25-7-11-27(12-8-25)21-37-31(41)3-1-5-33(43)39-23-29-15-17-30(18-16-29)24-40-34(44)6-2-4-32(42)38-22-28-13-9-26(20-36)10-14-28/h7-18H,1-6,19-24,35-36H2,(H,37,41)(H,38,42)(H,39,43)(H,40,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093144

(CHEMBL46120 | N-(4-Guanidino-benzyl)-N'-(4-{[2-(4-...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](\[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C30H36N10O4/c31-29(32)39-23-9-5-21(6-10-23)17-37-27(43)13-25(41)35-15-19-1-2-20(4-3-19)16-36-26(42)14-28(44)38-18-22-7-11-24(12-8-22)40-30(33)34/h1-12H,13-18H2,(H,35,41)(H,36,42)(H,37,43)(H,38,44)(H4,31,32,39)(H4,33,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093132

(CHEMBL75749 | [3-(4-Guanidino-benzyl)-ureido]-acet...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-[#6]-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#6]-[#7]-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](\[#7])-[#7])cc1 Show InChI InChI=1S/C30H42N10O6/c31-27(32)39-21-11-7-19(8-12-21)15-35-29(43)37-17-25(41)45-23-3-1-4-24(6-2-5-23)46-26(42)18-38-30(44)36-16-20-9-13-22(14-10-20)40-28(33)34/h7-14,23-24H,1-6,15-18H2,(H4,31,32,39)(H4,33,34,40)(H2,35,37,43)(H2,36,38,44)/t23-,24+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50084616

(CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...)Show SMILES NCc1ccc(CNC(=O)CNC(=O)OCc2ccc(COC(=O)NCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O6/c31-13-21-1-5-23(6-2-21)15-33-27(37)17-35-29(39)41-19-25-9-11-26(12-10-25)20-42-30(40)36-18-28(38)34-16-24-7-3-22(14-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093137

(CHEMBL80149 | [(S)-1-(4-Aminomethyl-benzylcarbamoy...)Show SMILES C[C@H](NC(=O)OCc1ccc(COC(=O)N[C@@H](C)C(=O)NCc2ccc(CN)cc2)cc1)C(=O)NCc1ccc(CN)cc1 Show InChI InChI=1S/C32H40N6O6/c1-21(29(39)35-17-25-7-3-23(15-33)4-8-25)37-31(41)43-19-27-11-13-28(14-12-27)20-44-32(42)38-22(2)30(40)36-18-26-9-5-24(16-34)6-10-26/h3-14,21-22H,15-20,33-34H2,1-2H3,(H,35,39)(H,36,40)(H,37,41)(H,38,42)/t21-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50093136

(CHEMBL309788 | N-(4-Guanidino-benzyl)-N'-(4-{[3-(4...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O4/c33-31(34)41-25-9-5-23(6-10-25)19-39-29(45)15-13-27(43)37-17-21-1-2-22(4-3-21)18-38-28(44)14-16-30(46)40-20-24-7-11-26(12-8-24)42-32(35)36/h1-12H,13-20H2,(H,37,43)(H,38,44)(H,39,45)(H,40,46)(H4,33,34,41)(H4,35,36,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against thrombin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50084616

(CHEMBL310290 | [(4-Aminomethyl-benzylcarbamoyl)-me...)Show SMILES NCc1ccc(CNC(=O)CNC(=O)OCc2ccc(COC(=O)NCC(=O)NCc3ccc(CN)cc3)cc2)cc1 Show InChI InChI=1S/C30H36N6O6/c31-13-21-1-5-23(6-2-21)15-33-27(37)17-35-29(39)41-19-25-9-11-26(12-10-25)20-42-30(40)36-18-28(38)34-16-24-7-3-22(14-32)4-8-24/h1-12H,13-20,31-32H2,(H,33,37)(H,34,38)(H,35,39)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093145

(CHEMBL408300 | [2-(4-Guanidino-benzylcarbamoyl)-et...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#7]-[#6](=O)-[#8]-[#6]-c2ccc(-[#6]-[#8]-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O6/c33-29(34)41-25-9-5-21(6-10-25)17-39-27(43)13-15-37-31(45)47-19-23-1-2-24(4-3-23)20-48-32(46)38-16-14-28(44)40-18-22-7-11-26(12-8-22)42-30(35)36/h1-12H,13-20H2,(H,37,45)(H,38,46)(H,39,43)(H,40,44)(H4,33,34,41)(H4,35,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093140

(CHEMBL43463 | Derivative of piperazine-1-carboxyli...)Show SMILES O=C(NCc1ccccc1)N1CCN(CC1)C(=O)O[C@H]1CCC[C@H](CCC1)OC(=O)N1CCN(CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C34H46N6O6/c41-31(35-25-27-9-3-1-4-10-27)37-17-21-39(22-18-37)33(43)45-29-13-7-15-30(16-8-14-29)46-34(44)40-23-19-38(20-24-40)32(42)36-26-28-11-5-2-6-12-28/h1-6,9-12,29-30H,7-8,13-26H2,(H,35,41)(H,36,42)/t29-,30+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50093136

(CHEMBL309788 | N-(4-Guanidino-benzyl)-N'-(4-{[3-(4...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c2ccc(-[#6]-[#7]-[#6](=O)-[#6]-[#6]-[#6](=O)-[#7]-[#6]-c3ccc(cc3)\[#7]=[#6](/[#7])-[#7])cc2)cc1 Show InChI InChI=1S/C32H40N10O4/c33-31(34)41-25-9-5-23(6-10-25)19-39-29(45)15-13-27(43)37-17-21-1-2-22(4-3-21)18-38-28(44)14-16-30(46)40-20-24-7-11-26(12-8-24)42-32(35)36/h1-12H,13-20H2,(H,37,43)(H,38,44)(H,39,45)(H,40,46)(H4,33,34,41)(H4,35,36,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory potency against plasmin |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |
Tryptase gamma
(Homo sapiens (Human)) | BDBM50093142

(1,5-di{4-[4-amino(imino)methylaminobenzylcarbamoyl...)Show SMILES [#7]\[#6](-[#7])=[#7]/c1ccc(-[#6]-[#7]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#8]-[#6@H]-2-[#6]-[#6]-[#6]-[#6@H](-[#6]-[#6]-[#6]-2)-[#8]-[#6](=O)-[#7]-2-[#6]-[#6]-[#7](-[#6]-[#6]-2)-[#6](=O)-[#7]-[#6]-c2ccc(cc2)\[#7]=[#6](/[#7])-[#7])cc1 Show InChI InChI=1S/C36H52N12O6/c37-31(38)43-27-11-7-25(8-12-27)23-41-33(49)45-15-19-47(20-16-45)35(51)53-29-3-1-4-30(6-2-5-29)54-36(52)48-21-17-46(18-22-48)34(50)42-24-26-9-13-28(14-10-26)44-32(39)40/h7-14,29-30H,1-6,15-24H2,(H,41,49)(H,42,50)(H4,37,38,43)(H4,39,40,44)/t29-,30+ | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Dissociation cosntant of compound was evaluated from its IC50 against tryptase at different concentrations |
Bioorg Med Chem Lett 10: 2357-60 (2001)
BindingDB Entry DOI: 10.7270/Q2NP23NX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data