

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50366760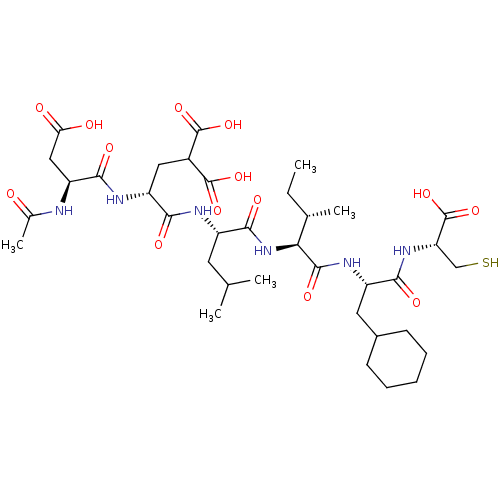 (CHEMBL2369589) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50366760 (CHEMBL2369589) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096410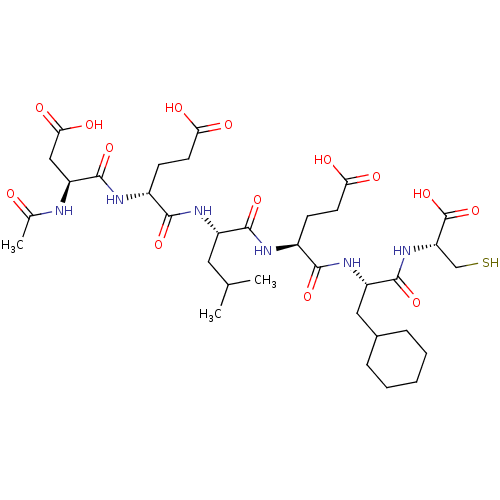 (AcAsp-D-Glu-Leu-Glu-Cha-Cys | CHEMBL60967) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50084685 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096411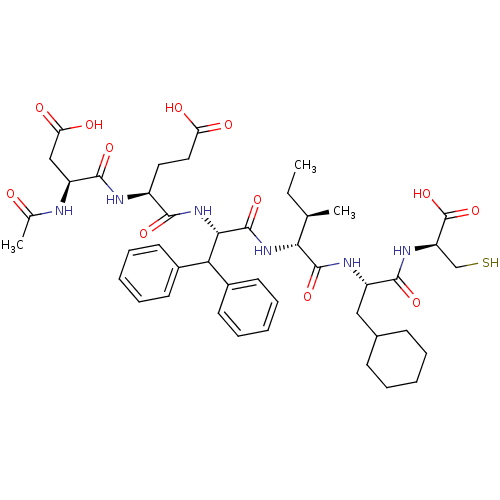 (AcAsp-Glu-Dif-Ile-Cha-Cys | CHEMBL61390) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50084685 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096404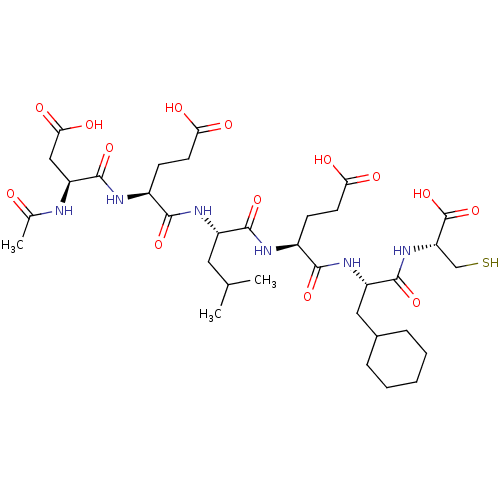 (AcAsp-Glu-Leu-Glu-Cha-Cys | CHEMBL305570) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096410 (AcAsp-D-Glu-Leu-Glu-Cha-Cys | CHEMBL60967) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096405 (AcAsp-Glu-Met-Glu-Nal-Cyse | CHEMBL64618) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096411 (AcAsp-Glu-Dif-Ile-Cha-Cys | CHEMBL61390) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096407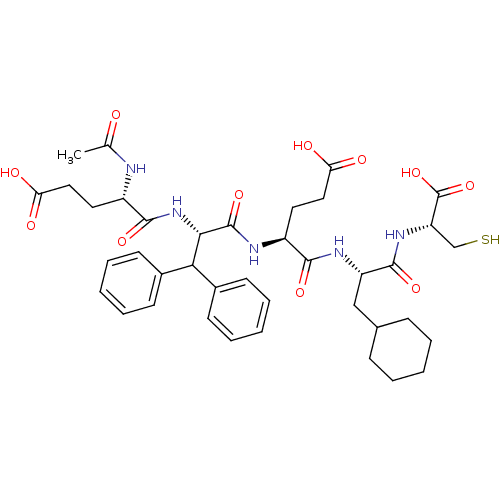 (AcGlu-Dif-Glu-Cha-Cys | CHEMBL431559) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50084634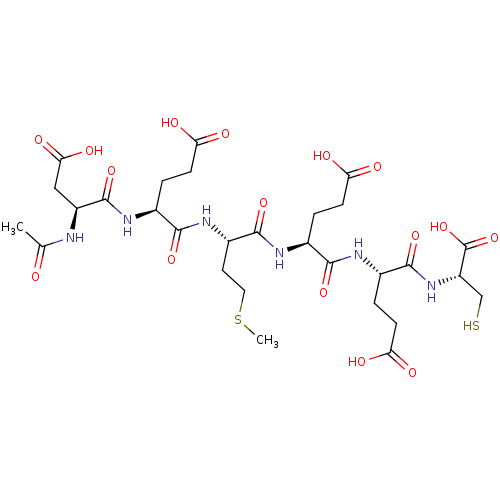 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096409 (AcAsp-Glu-Met-Glu-Cha-Cys | CHEMBL64867) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096409 (AcAsp-Glu-Met-Glu-Cha-Cys | CHEMBL64867) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096404 (AcAsp-Glu-Leu-Glu-Cha-Cys | CHEMBL305570) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50084634 (4-(2-Acetylamino-3-carboxy-propionylamino)-4-(1-{3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096407 (AcGlu-Dif-Glu-Cha-Cys | CHEMBL431559) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096412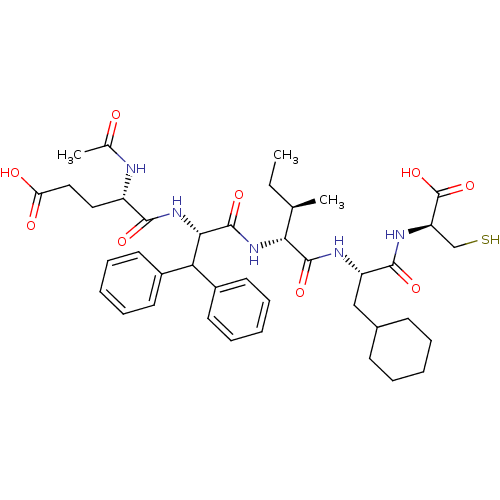 (AcGlu-Dif-Ile-Cha-Cys | CHEMBL304005) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096405 (AcAsp-Glu-Met-Glu-Nal-Cyse | CHEMBL64618) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096401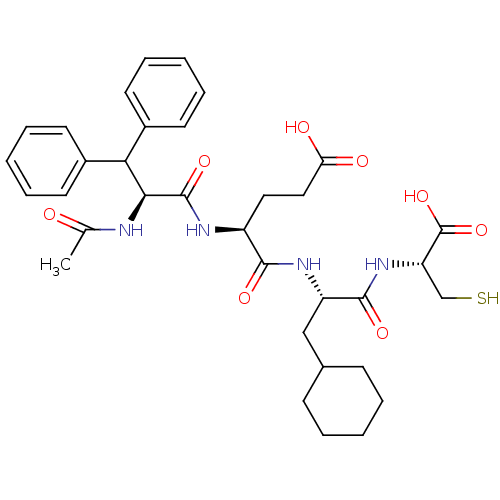 (AcDif-Glu-Cha-Cys | CHEMBL62433) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096412 (AcGlu-Dif-Ile-Cha-Cys | CHEMBL304005) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096413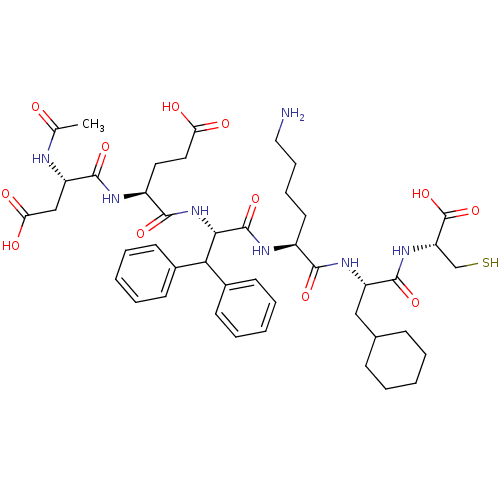 (AcAsp-Glu-Dif-Lys-Cha-Cys | CHEMBL294440) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096402 (Asp-D-Glu-Leu-Glu-Cha-Cys | CHEMBL303541) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096408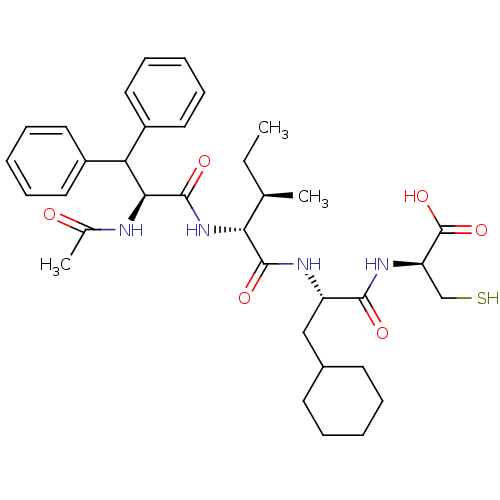 (AcDif-Ile-Cha-Cys | CHEMBL293631) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096406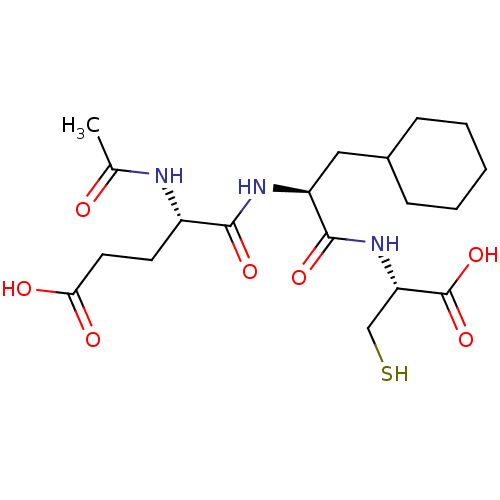 (Ac-Glu-Cha-Cys | CHEMBL303340) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096401 (AcDif-Glu-Cha-Cys | CHEMBL62433) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096403 (AcIle-Cha-Cys | CHEMBL60512) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (full-length) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096408 (AcDif-Ile-Cha-Cys | CHEMBL293631) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50096406 (Ac-Glu-Cha-Cys | CHEMBL303340) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease (isolated domain) | Bioorg Med Chem Lett 11: 203-6 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||