

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50083680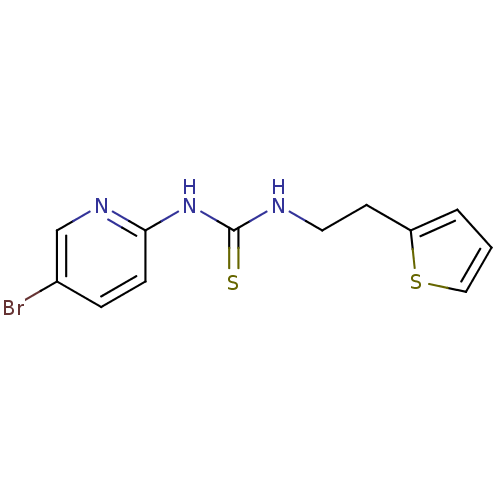 (1-(5-Bromo-pyridin-2-yl)-3-(2-thiophen-2-yl-ethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097041 (1-(5-Bromo-pyridin-2-yl)-3-[2-(4-hydroxy-phenyl)-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097038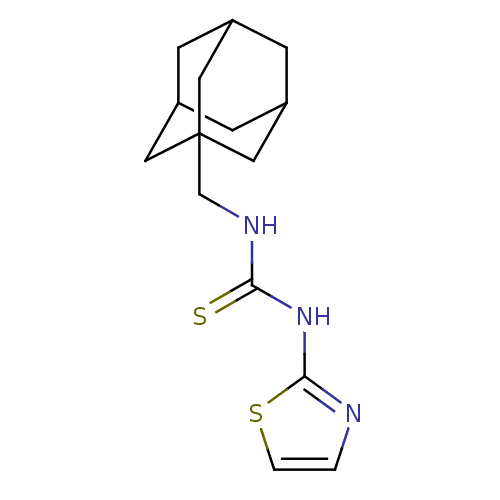 (1-Adamantan-1-ylmethyl-3-thiazol-2-yl-thiourea | C...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097046 (1-Furan-2-ylmethyl-3-thiazol-2-yl-thiourea | CHEMB...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097044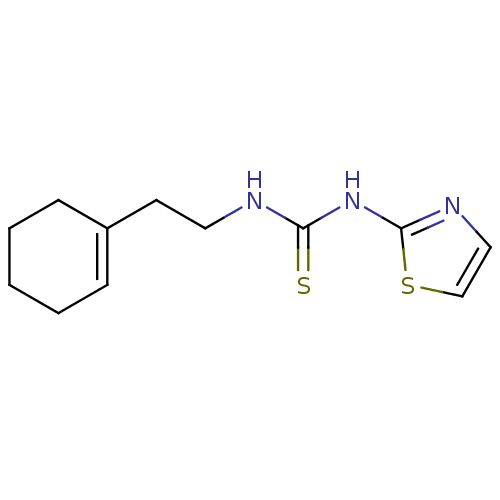 (1-(2-Cyclohex-1-enyl-ethyl)-3-thiazol-2-yl-thioure...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097038 (1-Adamantan-1-ylmethyl-3-thiazol-2-yl-thiourea | C...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was tested for agonistic activity against 5-HT uptake | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097039 (1-(1-Phenyl-propyl)-3-thiazol-2-yl-thiourea | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097046 (1-Furan-2-ylmethyl-3-thiazol-2-yl-thiourea | CHEMB...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097039 (1-(1-Phenyl-propyl)-3-thiazol-2-yl-thiourea | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097042 (1-[2-(1H-Indol-3-yl)-ethyl]-3-thiazol-2-yl-thioure...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50083680 (1-(5-Bromo-pyridin-2-yl)-3-(2-thiophen-2-yl-ethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097042 (1-[2-(1H-Indol-3-yl)-ethyl]-3-thiazol-2-yl-thioure...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097037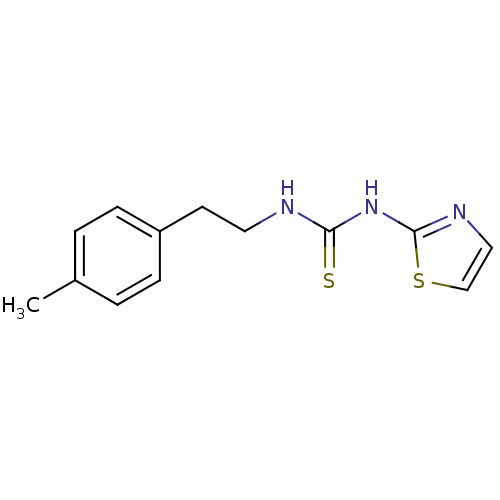 (1-Thiazol-2-yl-3-(2-p-tolyl-ethyl)-thiourea | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097040 (1-(2-Phenoxy-ethyl)-3-thiazol-2-yl-thiourea | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097047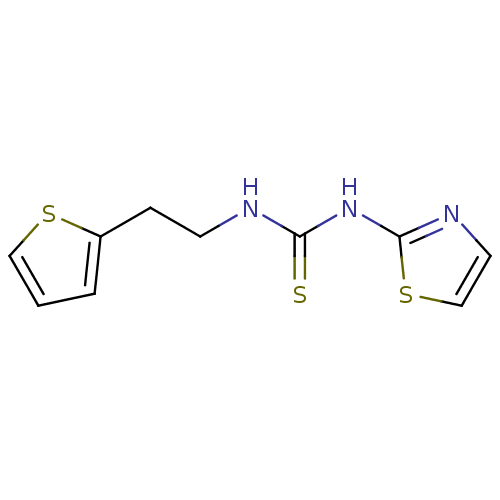 (1-Thiazol-2-yl-3-(2-thiophen-2-yl-ethyl)-thiourea ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in reverse tran... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097041 (1-(5-Bromo-pyridin-2-yl)-3-[2-(4-hydroxy-phenyl)-e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097044 (1-(2-Cyclohex-1-enyl-ethyl)-3-thiazol-2-yl-thioure...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1944 (BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 variant with Y181C plus K103N mutatio... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097037 (1-Thiazol-2-yl-3-(2-p-tolyl-ethyl)-thiourea | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097049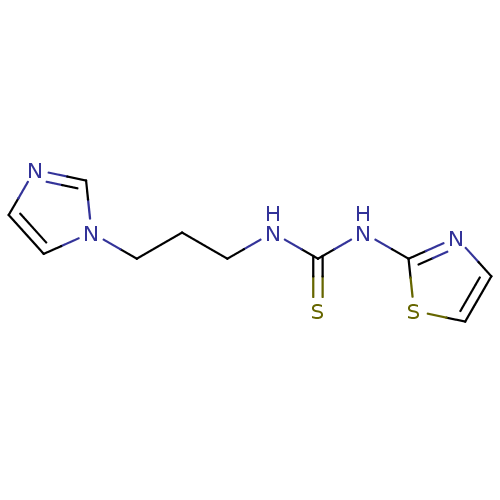 (1-(3-Imidazol-1-yl-propyl)-3-thiazol-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Inhibitory activity against non-nucleoside reverse transcriptase inhibitors (NNRTI) -resistant HIV-1 strain A17 with a Y181C mutation in RT (reverse ... | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097049 (1-(3-Imidazol-1-yl-propyl)-3-thiazol-2-yl-thiourea...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Compound was tested for binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097040 (1-(2-Phenoxy-ethyl)-3-thiazol-2-yl-thiourea | CHEM...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 11: 523-8 (2001) BindingDB Entry DOI: 10.7270/Q237780X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||