

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM2483 ((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097572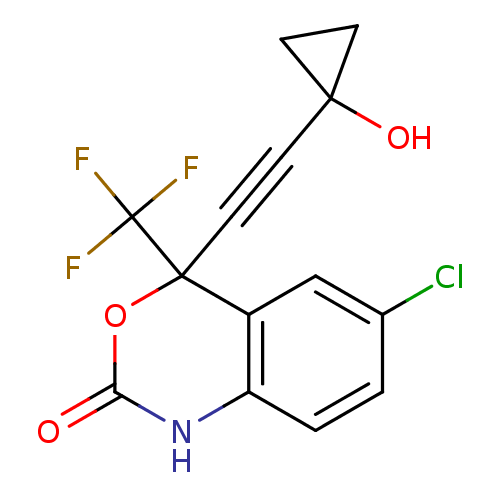 (6-Chloro-4-(1-hydroxy-cyclopropylethynyl)-4-triflu...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097571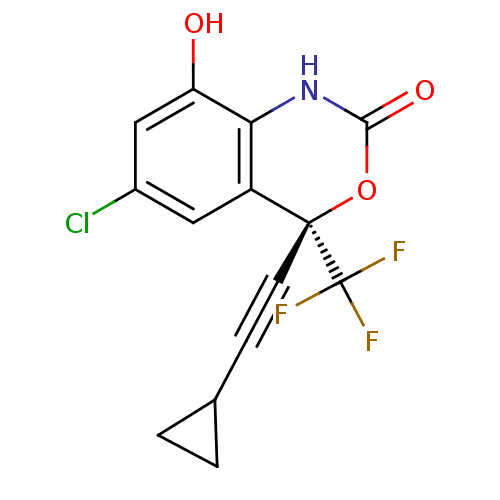 ((S)-6-Chloro-4-cyclopropylethynyl-8-hydroxy-4-trif...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097575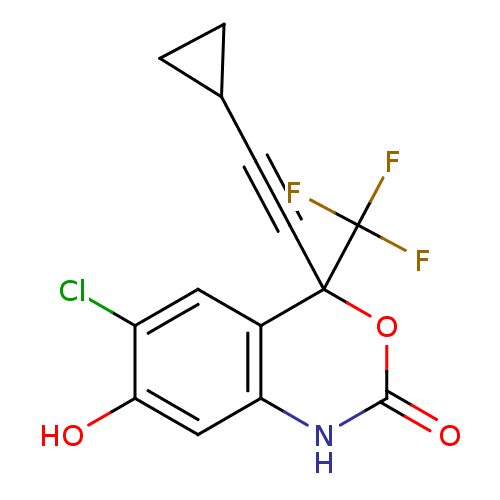 (6-Chloro-4-cyclopropylethynyl-7-hydroxy-4-trifluor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097573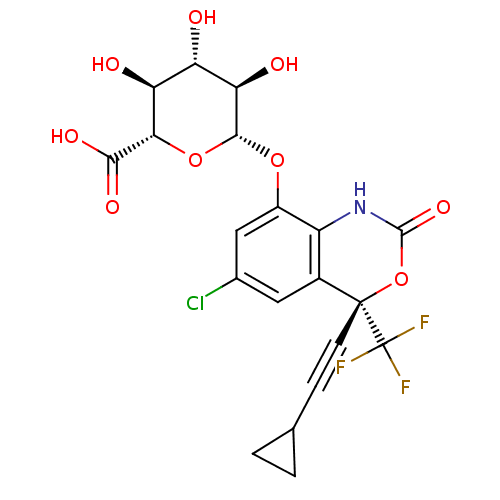 ((2S,3S,4S,5R,6S)-6-((S)-6-Chloro-4-cyclopropylethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem | PubMed | n/a | n/a | 9.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097576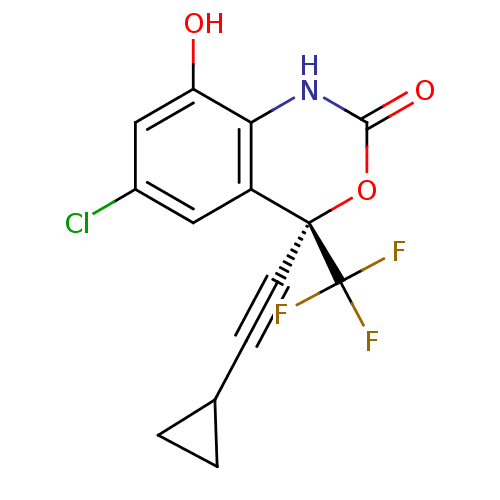 ((S)-6-Chloro-4-cyclopropylethynyl-8-hydroxy-4-trif...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50097574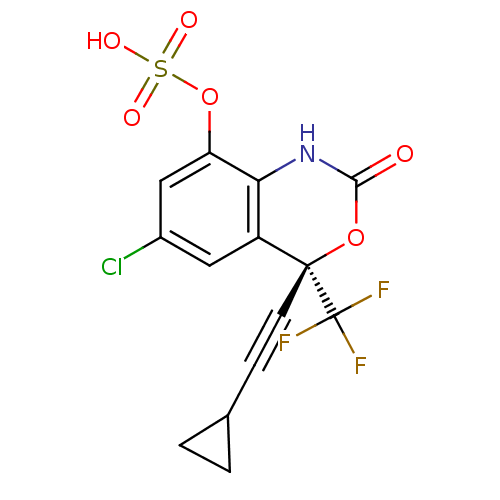 (CHEMBL346585 | Sulfuric acid mono-((S)-6-chloro-4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Pharmaceuticals Company Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | Bioorg Med Chem Lett 11: 619-22 (2001) BindingDB Entry DOI: 10.7270/Q2BC403C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||