

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50098216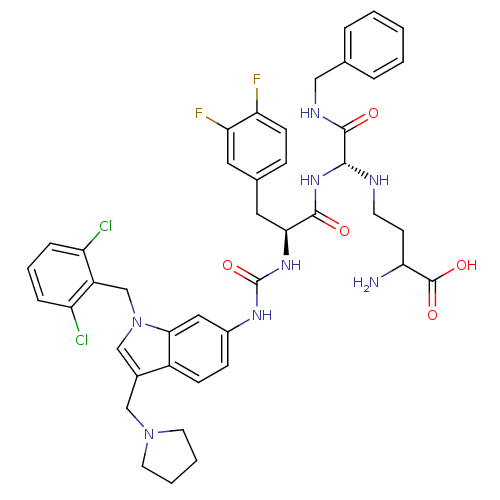 (2-Amino-4-({benzylcarbamoyl-[2-{3-[1-(2,6-dichloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098212 ((S)-2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098211 ((S)-2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrroli...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098214 (2-[2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098218 ((S)-2-[(S)-2-{3-[1-(4-Fluoro-benzyl)-3-pyrrolidin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098213 (2-(2-{3-[1-(4-Fluoro-benzyl)-3-pyrrolidin-1-ylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098217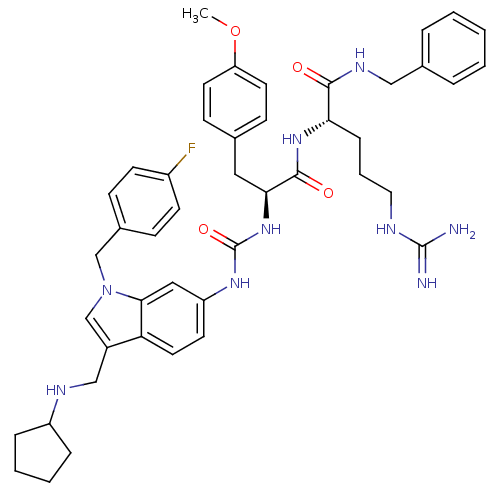 (2-[2-{3-[3-Cyclopentylaminomethyl-1-(4-fluoro-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50098210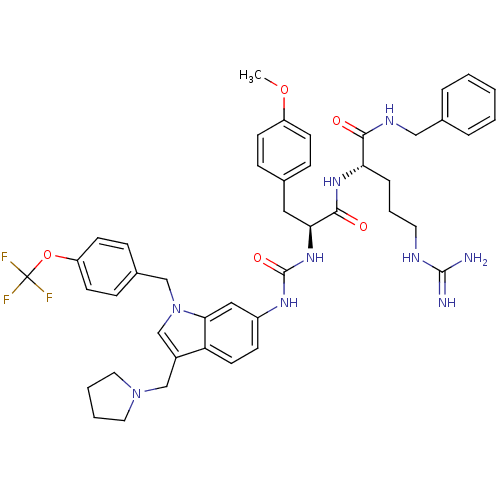 (5-Guanidino-2-(3-(4-methoxy-phenyl)-2-{3-[3-pyrrol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonist activity against Thrombin induced gel-filtered platelet (GFP) aggregation at 0.15 nM. | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098215 (4-Amino-N-benzyl-2-[2-{3-[1-(2,6-dichloro-benzyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding Affinity of ligand against Protease-activated Receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098212 ((S)-2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098210 (5-Guanidino-2-(3-(4-methoxy-phenyl)-2-{3-[3-pyrrol...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098217 (2-[2-{3-[3-Cyclopentylaminomethyl-1-(4-fluoro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 10.7 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098214 (2-[2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrrolidin-1-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098216 (2-Amino-4-({benzylcarbamoyl-[2-{3-[1-(2,6-dichloro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098213 (2-(2-{3-[1-(4-Fluoro-benzyl)-3-pyrrolidin-1-ylmeth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098218 ((S)-2-[(S)-2-{3-[1-(4-Fluoro-benzyl)-3-pyrrolidin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50098211 ((S)-2-[(S)-2-{3-[1-(2,6-Dichloro-benzyl)-3-pyrroli...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
The R. W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Binding affinity against Protease-activated receptor (PAR-1) using [3H]-s-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2, 10 nM (Kd= 15 nM) | J Med Chem 44: 1021-4 (2001) BindingDB Entry DOI: 10.7270/Q24X571D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||