

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50099151 (CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50099151 (CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50099160 (CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50099160 (CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50099150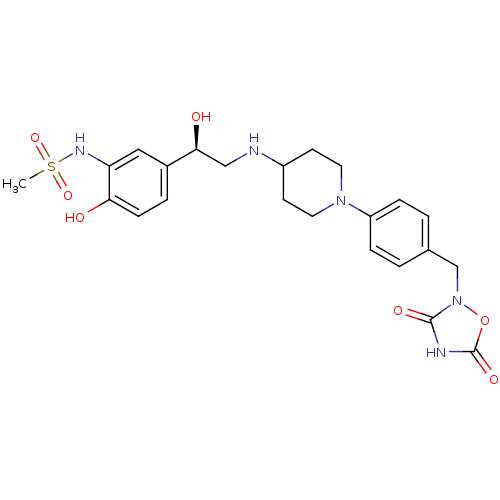 (CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50099150 (CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of 125 I-Iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099156 (CHEMBL174534 | N'-(5-{4-[4-((S)-2-Hydroxy-3-phenox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 940 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099157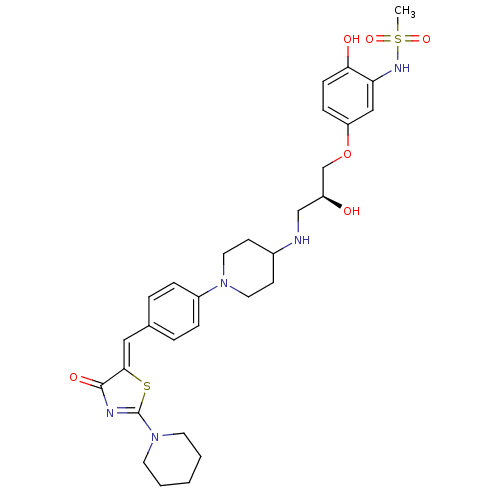 (CHEMBL435307 | N-[2-Hydroxy-5-((S)-2-hydroxy-3-{1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099160 (CHEMBL354906 | [5-(4-{4-[(R)-2-Hydroxy-2-(4-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099168 (CHEMBL177400 | N-{5-[(R)-2-(1-{4-[2-(N',N'-Dimethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099155 (CHEMBL174054 | N-[2-Hydroxy-5-(1-hydroxy-2-{1-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50097798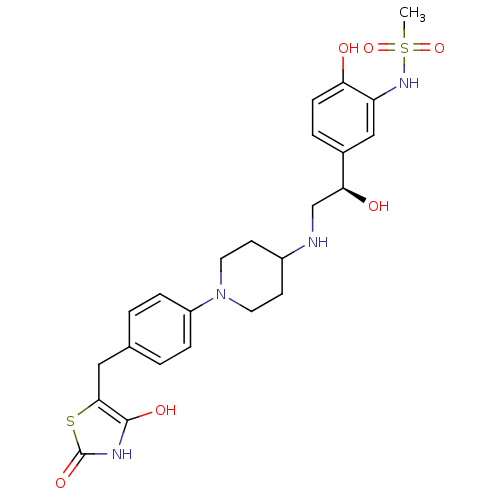 (CHEMBL163262 | N-[5-((R)-2-{1-[4-(2,4-Dioxo-thiazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity as cAMP accumulation in CHO cells | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099165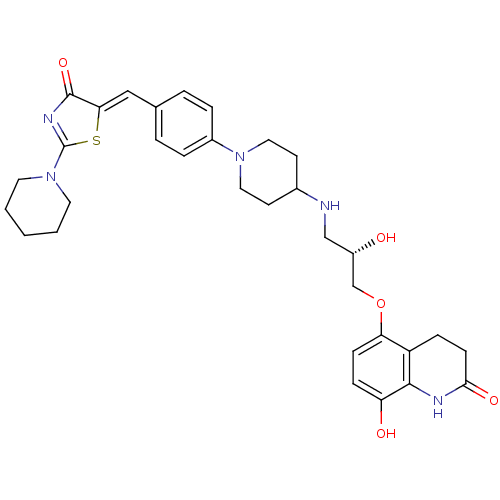 (8-Hydroxy-5-((S)-2-hydroxy-3-{1-[4-(4-oxo-2-piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099167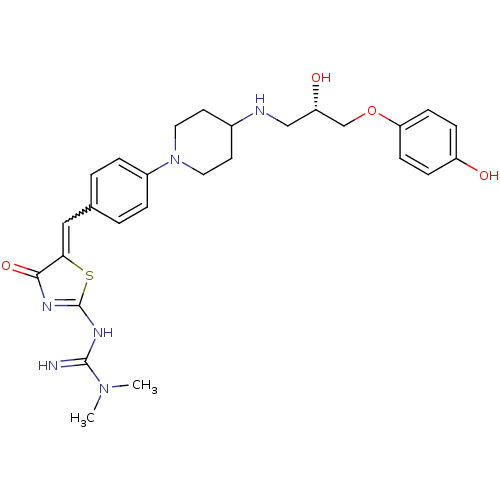 (CHEMBL369155 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099159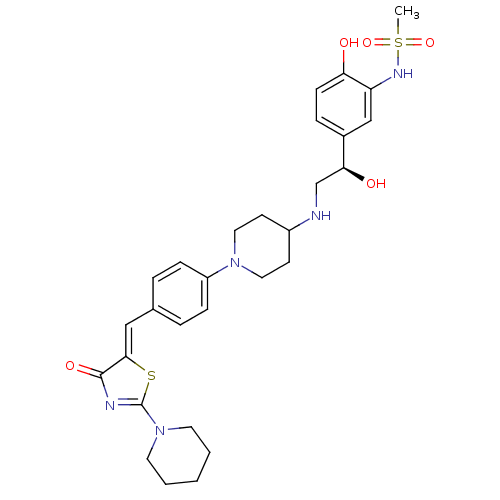 (CHEMBL174442 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099164 (CHEMBL366657 | N-[5-((R)-2-{1-[4-(2-Cyanoamino-4-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099166 (CHEMBL367561 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(8-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099149 (5-{4-[4-((S)-2-Hydroxy-3-phenoxy-propylamino)-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099161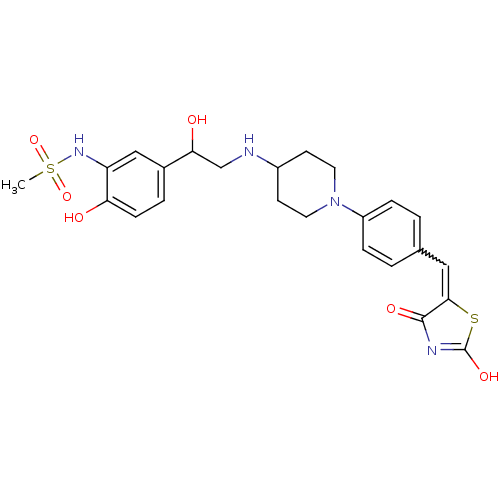 (CHEMBL177185 | N-[5-(2-{1-[4-(2,4-Dioxo-thiazolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099162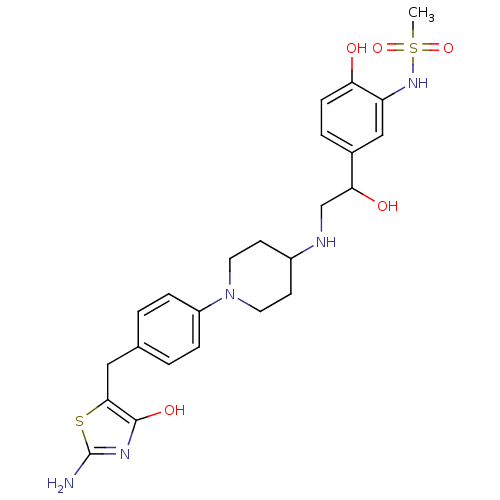 (CHEMBL177438 | N-[2-Hydroxy-5-(1-hydroxy-2-{1-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099150 (CHEMBL368584 | N-[5-(2-{1-[4-((R)-3,5-Dioxo-[1,2,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099154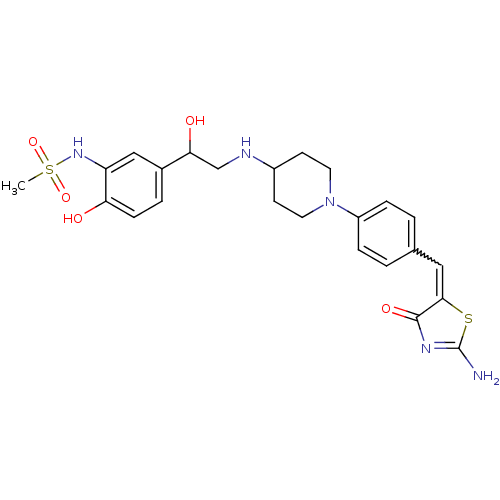 (CHEMBL173472 | N-[2-Hydroxy-5-(1-hydroxy-2-{1-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099151 (CHEMBL177442 | N'-[5-(4-{4-[(S)-2-Hydroxy-3-(2-oxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099158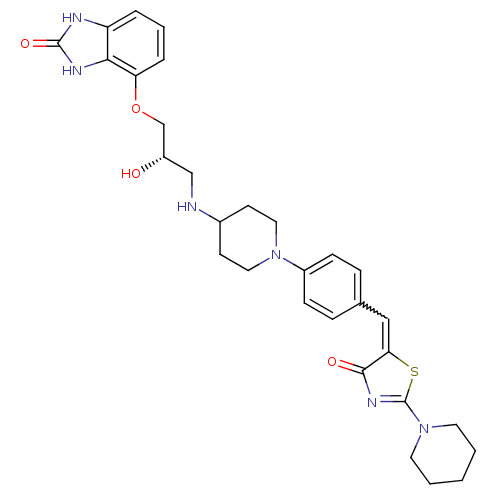 (4-((S)-2-Hydroxy-3-{1-[4-(4-oxo-2-piperidin-1-yl-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099163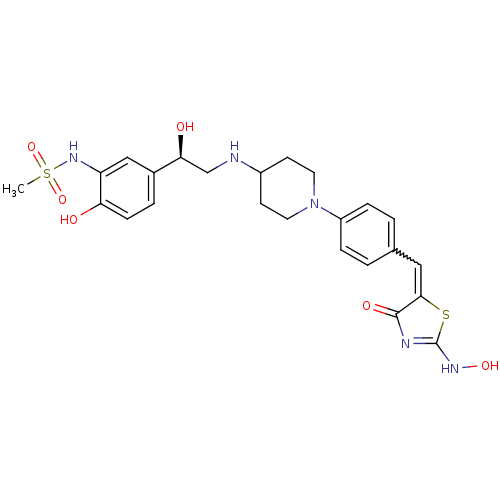 (CHEMBL178848 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 80 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099152 (CHEMBL366395 | N-{5-[(R)-2-(1-{4-[2-(1-Benzyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 90 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50099153 (CHEMBL451185 | N-[2-Hydroxy-5-(1-hydroxy-2-{1-[4-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Beta 3-adrenergic receptor agonistic activity, evaluated by measurement of cAMP accumulation in CHO cells. | Bioorg Med Chem Lett 11: 981-4 (2001) BindingDB Entry DOI: 10.7270/Q2125RZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||