

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin G (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human cathepsin G | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50101136 (1,3-Bis-benzo[1,3]dioxol-5-ylmethyl-[1,3]diazetidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50101135 (1,3-Bis-(4-methoxy-benzyl)-[1,3]diazetidine-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50101131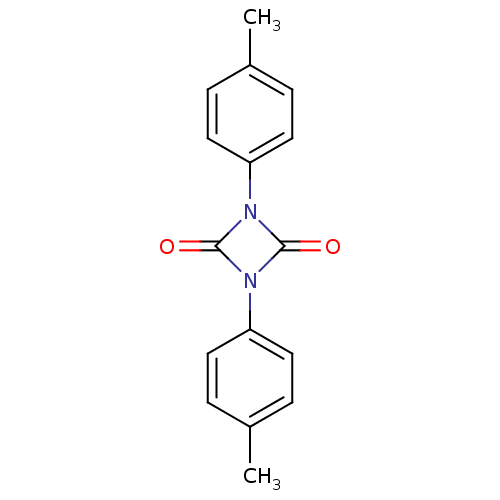 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human cathepsin G | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50101137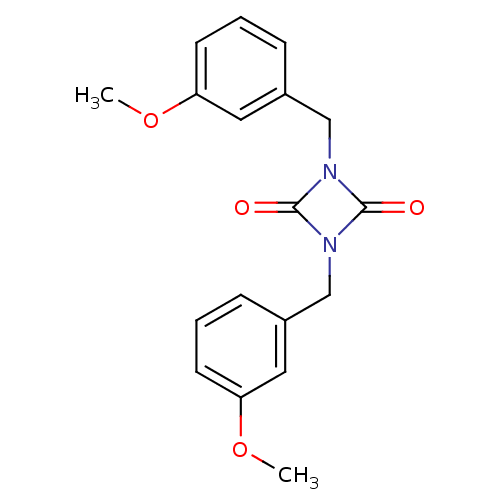 (1,3-Bis-(3-methoxy-benzyl)-[1,3]diazetidine-2,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50101131 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic trypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50101134 (1,3-Bis-benzo[1,3]dioxol-5-yl-[1,3]diazetidine-2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human neutrophil elastase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human neutrophil elastase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymase (Homo sapiens (Human)) | BDBM50101131 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human Serine protease chymase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50101131 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic trypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50101131 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human neutrophil elastase | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic alpha-chymotrypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human cathepsin G | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101131 (1,3-Di-p-tolyl-[1,3]diazetidine-2,4-dione | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50101132 (1,3-Dibenzyl-[1,3]diazetidine-2,4-dione | CHEMBL47...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human plasmin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human thrombin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against human plasmin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM50101133 (1,3-Diethyl-[1,3]diazetidine-2,4-dione | CHEMBL298...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd. Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against bovine pancreatic trypsin | Bioorg Med Chem Lett 11: 1691-4 (2001) BindingDB Entry DOI: 10.7270/Q2V12421 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||