 Found 14 hits of Enzyme Inhibition Constant Data
Found 14 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50366682
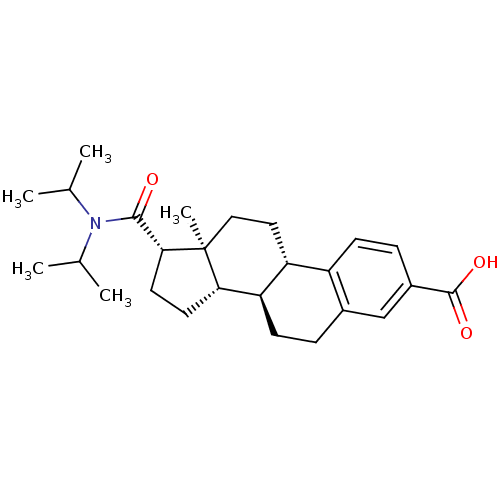
(CHEMBL1627395)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CC[C@H]2[C@@H]3CCc4cc(ccc4[C@H]3CC[C@]12C)C(O)=O Show InChI InChI=1S/C26H37NO3/c1-15(2)27(16(3)4)24(28)23-11-10-22-21-9-6-17-14-18(25(29)30)7-8-19(17)20(21)12-13-26(22,23)5/h7-8,14-16,20-23H,6,9-13H2,1-5H3,(H,29,30)/t20-,21-,22+,23-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101143
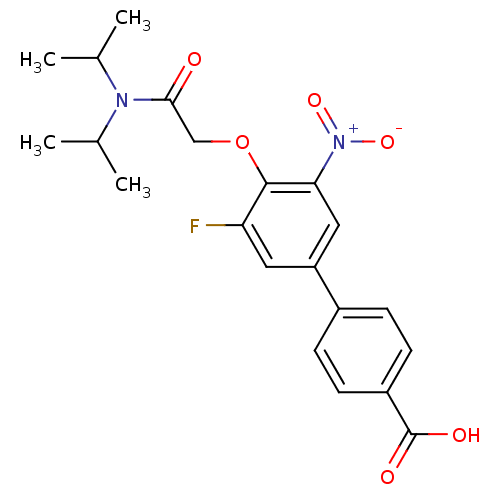
(4'-[(Diisopropylcarbamoyl)-methoxy]-5'-fluoro-3'-n...)Show SMILES CC(C)N(C(C)C)C(=O)COc1c(F)cc(cc1[N+]([O-])=O)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H23FN2O6/c1-12(2)23(13(3)4)19(25)11-30-20-17(22)9-16(10-18(20)24(28)29)14-5-7-15(8-6-14)21(26)27/h5-10,12-13H,11H2,1-4H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101145

((4aR,6aS,7S,11aR)-4a,6a-Dimethyl-2-oxo-hexadecahyd...)Show SMILES CC(C)N(C(C)C)C(=O)[C@H]1CCC2C3CC[C@H]4NC(=O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C25H42N2O2/c1-15(2)27(16(3)4)23(29)20-9-8-18-17-7-10-21-25(6,14-12-22(28)26-21)19(17)11-13-24(18,20)5/h15-21H,7-14H2,1-6H3,(H,26,28)/t17?,18?,19?,20-,21-,24+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101155
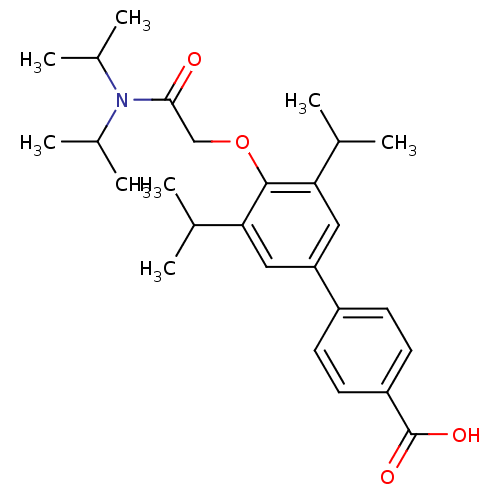
(4'-[(Diisopropylcarbamoyl)-methoxy]-3',5'-diisopro...)Show SMILES CC(C)N(C(C)C)C(=O)COc1c(cc(cc1C(C)C)-c1ccc(cc1)C(O)=O)C(C)C Show InChI InChI=1S/C27H37NO4/c1-16(2)23-13-22(20-9-11-21(12-10-20)27(30)31)14-24(17(3)4)26(23)32-15-25(29)28(18(5)6)19(7)8/h9-14,16-19H,15H2,1-8H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101154

(5'-Fluoro-3'-nitro-4'-[(trityl-carbamoyl)-methoxy]...)Show SMILES OC(=O)c1ccc(cc1)-c1cc(F)c(OCC(=O)NC(c2ccccc2)(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H25FN2O6/c35-29-20-25(23-16-18-24(19-17-23)33(39)40)21-30(37(41)42)32(29)43-22-31(38)36-34(26-10-4-1-5-11-26,27-12-6-2-7-13-27)28-14-8-3-9-15-28/h1-21H,22H2,(H,36,38)(H,39,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101153
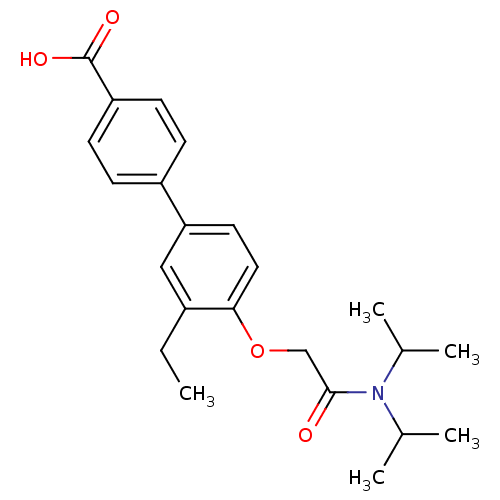
(4'-[(Diisopropylcarbamoyl)-methoxy]-3'-ethyl-biphe...)Show SMILES CCc1cc(ccc1OCC(=O)N(C(C)C)C(C)C)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H29NO4/c1-6-17-13-20(18-7-9-19(10-8-18)23(26)27)11-12-21(17)28-14-22(25)24(15(2)3)16(4)5/h7-13,15-16H,6,14H2,1-5H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101146
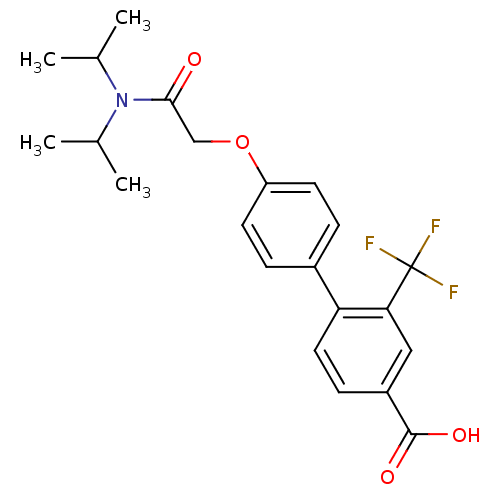
(4'-[(Diisopropylcarbamoyl)-methoxy]-2-trifluoromet...)Show SMILES CC(C)N(C(C)C)C(=O)COc1ccc(cc1)-c1ccc(cc1C(F)(F)F)C(O)=O Show InChI InChI=1S/C22H24F3NO4/c1-13(2)26(14(3)4)20(27)12-30-17-8-5-15(6-9-17)18-10-7-16(21(28)29)11-19(18)22(23,24)25/h5-11,13-14H,12H2,1-4H3,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101149

(4'-[(Diisopropylcarbamoyl)-methoxy]-biphenyl-4-car...)Show SMILES CC(C)N(C(C)C)C(=O)COc1ccc(cc1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H25NO4/c1-14(2)22(15(3)4)20(23)13-26-19-11-9-17(10-12-19)16-5-7-18(8-6-16)21(24)25/h5-12,14-15H,13H2,1-4H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101156

(2-Chloro-4'-[(diisopropylcarbamoyl)-methoxy]-biphe...)Show SMILES CC(C)N(C(C)C)C(=O)COc1ccc(cc1)-c1ccc(cc1Cl)C(O)=O Show InChI InChI=1S/C21H24ClNO4/c1-13(2)23(14(3)4)20(24)12-27-17-8-5-15(6-9-17)18-10-7-16(21(25)26)11-19(18)22/h5-11,13-14H,12H2,1-4H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101144
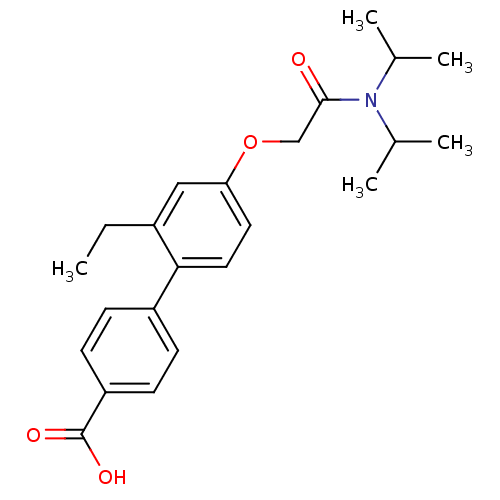
(4'-[(Diisopropylcarbamoyl)-methoxy]-2'-ethyl-biphe...)Show SMILES CCc1cc(OCC(=O)N(C(C)C)C(C)C)ccc1-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H29NO4/c1-6-17-13-20(28-14-22(25)24(15(2)3)16(4)5)11-12-21(17)18-7-9-19(10-8-18)23(26)27/h7-13,15-16H,6,14H2,1-5H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of type 2 5-alpha-reductase of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101148

(4'-[(Diisopropylcarbamoyl)-methoxy]-2,6-difluoro-b...)Show SMILES CC(C)N(C(C)C)C(=O)COc1ccc(cc1)-c1c(F)cc(cc1F)C(O)=O Show InChI InChI=1S/C21H23F2NO4/c1-12(2)24(13(3)4)19(25)11-28-16-7-5-14(6-8-16)20-17(22)9-15(21(26)27)10-18(20)23/h5-10,12-13H,11H2,1-4H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101150

(2',6'-Dichloro-4'-[(diisopropylcarbamoyl)-methoxy]...)Show SMILES CC(C)N(C(C)C)C(=O)COc1cc(Cl)c(c(Cl)c1)-c1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H23Cl2NO4/c1-12(2)24(13(3)4)19(25)11-28-16-9-17(22)20(18(23)10-16)14-5-7-15(8-6-14)21(26)27/h5-10,12-13H,11H2,1-4H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101147
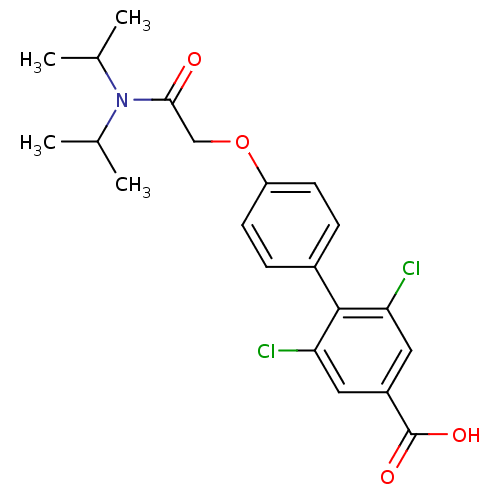
(2,6-Dichloro-4'-[(diisopropylcarbamoyl)-methoxy]-b...)Show SMILES CC(C)N(C(C)C)C(=O)COc1ccc(cc1)-c1c(Cl)cc(cc1Cl)C(O)=O Show InChI InChI=1S/C21H23Cl2NO4/c1-12(2)24(13(3)4)19(25)11-28-16-7-5-14(6-8-16)20-17(22)9-15(21(26)27)10-18(20)23/h5-10,12-13H,11H2,1-4H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50101151
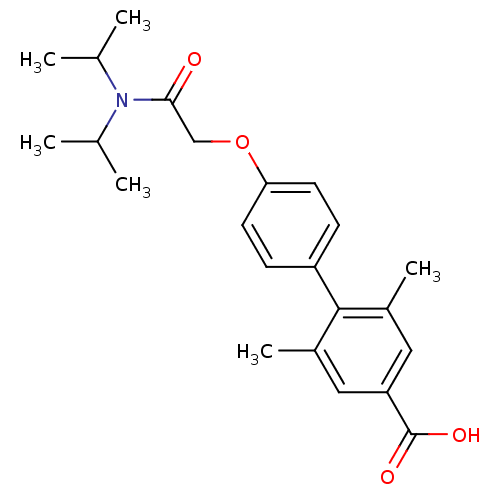
(4'-[(Diisopropylcarbamoyl)-methoxy]-2,6-dimethyl-b...)Show SMILES CC(C)N(C(C)C)C(=O)COc1ccc(cc1)-c1c(C)cc(cc1C)C(O)=O Show InChI InChI=1S/C23H29NO4/c1-14(2)24(15(3)4)21(25)13-28-20-9-7-18(8-10-20)22-16(5)11-19(23(26)27)12-17(22)6/h7-12,14-15H,13H2,1-6H3,(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Steroid 5-alpha-reductase type 2 of human prostates |
Bioorg Med Chem Lett 11: 1713-6 (2001)
BindingDB Entry DOI: 10.7270/Q2ZC83C9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data