 Found 24 hits of Enzyme Inhibition Constant Data
Found 24 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102880
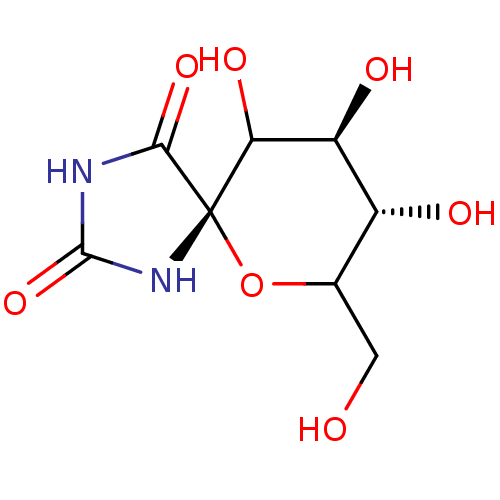
(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102880

(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50263769
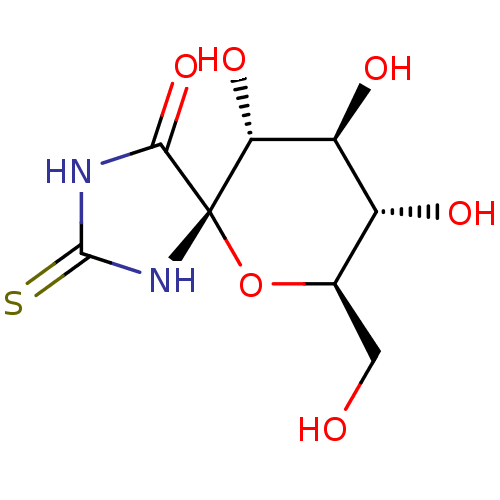
((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50263769

((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against liver Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50263769

((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase a |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102880

(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against liver Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102880

(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against liver Glycogen Phosphorylase a |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102880

(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase a |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50263769

((5S,7R,8S,9S,10R)-8,9,10-Trihydroxy-7-hydroxymethy...)Show SMILES OC[C@H]1O[C@@]2(NC(=S)NC2=O)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C8H12N2O6S/c11-1-2-3(12)4(13)5(14)8(16-2)6(15)9-7(17)10-8/h2-5,11-14H,1H2,(H2,9,10,15,17)/t2-,3-,4+,5-,8+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 2.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against liver Glycogen Phosphorylase a |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50240802
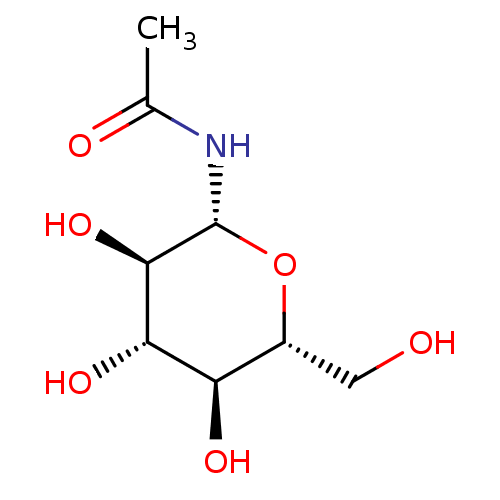
(1-N-ACETYL-BETA-D-GLUCOSAMINE | CHEMBL335315 | N-(...)Show SMILES CC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C8H15NO6/c1-3(11)9-8-7(14)6(13)5(12)4(2-10)15-8/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6+,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50240802

(1-N-ACETYL-BETA-D-GLUCOSAMINE | CHEMBL335315 | N-(...)Show SMILES CC(=O)N[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C8H15NO6/c1-3(11)9-8-7(14)6(13)5(12)4(2-10)15-8/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6+,7-,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50240801
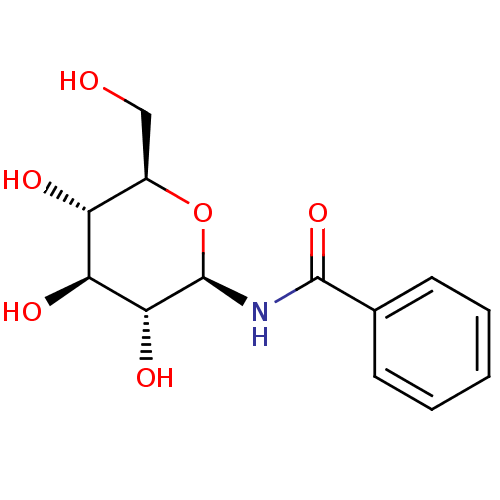
(CHEMBL131967 | N-((2R,3R,4S,5S,6R)-3,4,5-trihydrox...)Show SMILES OC[C@H]1O[C@@H](NC(=O)c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H17NO6/c15-6-8-9(16)10(17)11(18)13(20-8)14-12(19)7-4-2-1-3-5-7/h1-5,8-11,13,15-18H,6H2,(H,14,19)/t8-,9-,10+,11-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102884
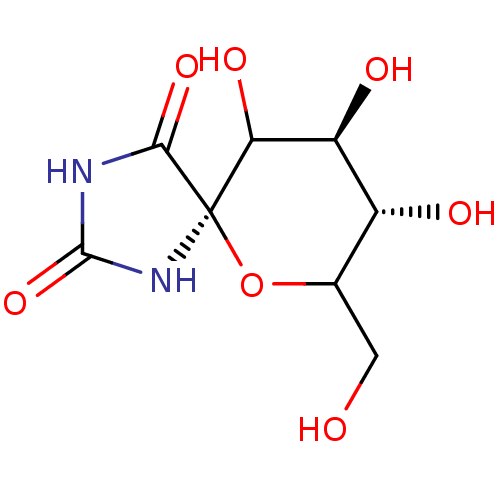
(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102884

(8,9,10-Trihydroxy-7-hydroxymethyl-6-oxa-1,3-diaza-...)Show SMILES OCC1O[C@]2(NC(=O)NC2=O)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12N2O7/c11-1-2-3(12)4(13)5(14)8(17-2)6(15)9-7(16)10-8/h2-5,11-14H,1H2,(H2,9,10,15,16)/t2?,3-,4+,5?,8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50240801

(CHEMBL131967 | N-((2R,3R,4S,5S,6R)-3,4,5-trihydrox...)Show SMILES OC[C@H]1O[C@@H](NC(=O)c2ccccc2)[C@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C13H17NO6/c15-6-8-9(16)10(17)11(18)13(20-8)14-12(19)7-4-2-1-3-5-7/h1-5,8-11,13,15-18H,6H2,(H,14,19)/t8-,9-,10+,11-,13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 1.44E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102891

(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-pyran-...)Show InChI InChI=1S/C7H13NO6/c8-7(13)6-5(12)4(11)3(10)2(1-9)14-6/h2-6,9-12H,1H2,(H2,8,13)/t2?,3-,4+,5?,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102890
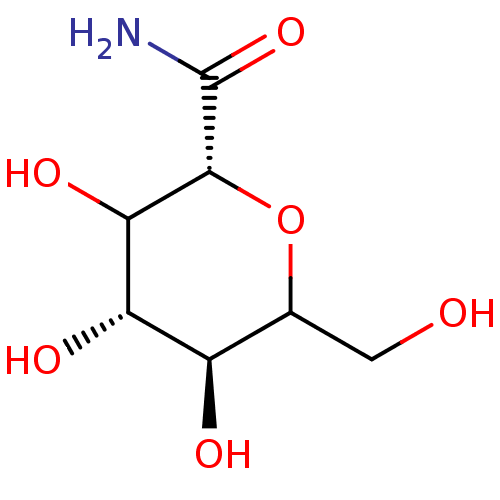
(3,4,5-Trihydroxy-6-hydroxymethyl-tetrahydro-pyran-...)Show InChI InChI=1S/C7H13NO6/c8-7(13)6-5(12)4(11)3(10)2(1-9)14-6/h2-6,9-12H,1H2,(H2,8,13)/t2?,3-,4+,5?,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102885
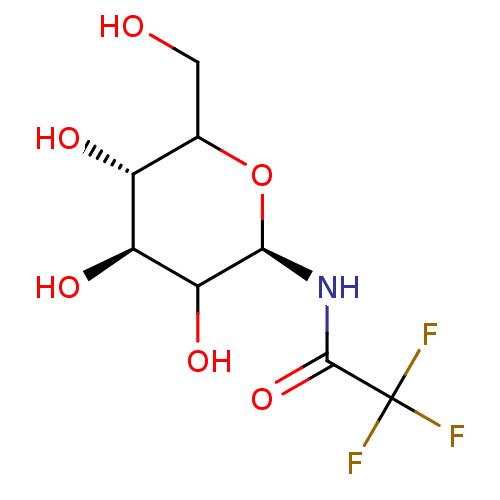
(2,2,2-Trifluoro-N-(3,4,5-trihydroxy-6-hydroxymethy...)Show SMILES OCC1O[C@@H](NC(=O)C(F)(F)F)C(O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C8H12F3NO6/c9-8(10,11)7(17)12-6-5(16)4(15)3(14)2(1-13)18-6/h2-6,13-16H,1H2,(H,12,17)/t2?,3-,4+,5?,6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102882
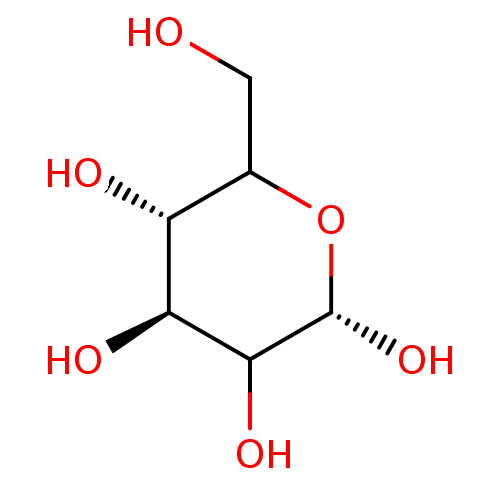
(6-Hydroxymethyl-tetrahydro-pyran-2,3,4,5-tetraol |...)Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2?,3-,4+,5?,6+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102888
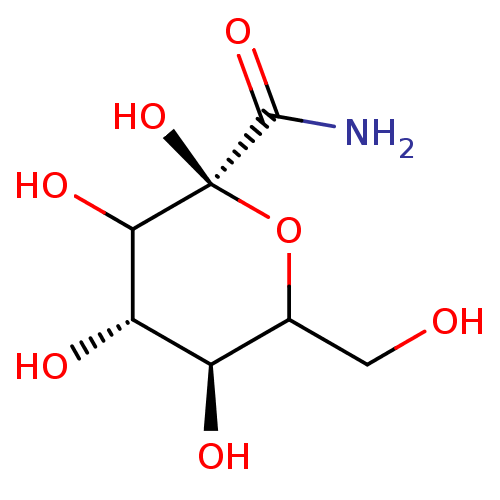
(2,3,4,5-Tetrahydroxy-6-hydroxymethyl-tetrahydro-py...)Show InChI InChI=1S/C7H13NO7/c8-6(13)7(14)5(12)4(11)3(10)2(1-9)15-7/h2-5,9-12,14H,1H2,(H2,8,13)/t2?,3-,4+,5?,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM34103
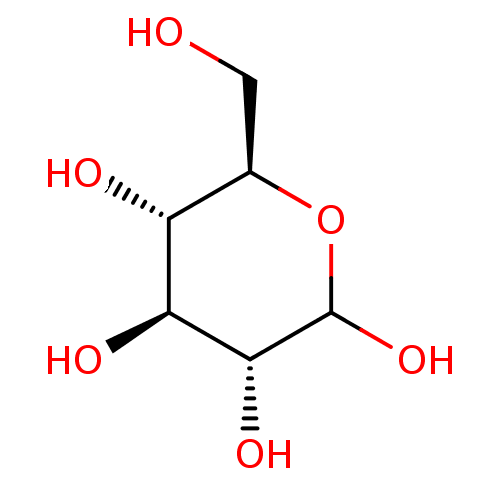
(D-glucose | dextrose | glucose)Show InChI InChI=1S/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 7.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102892
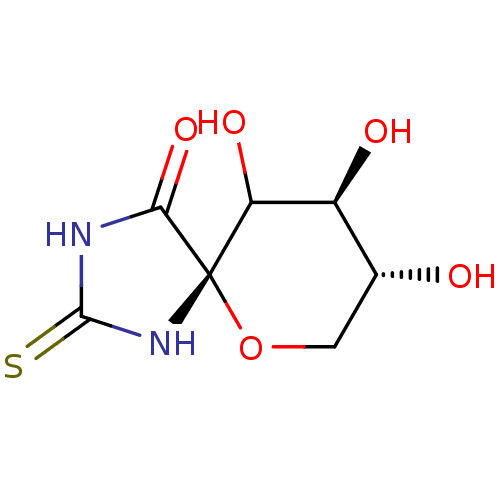
(8,9,10-Trihydroxy-2-thioxo-6-oxa-1,3-diaza-spiro[4...)Show InChI InChI=1S/C7H10N2O5S/c10-2-1-14-7(4(12)3(2)11)5(13)8-6(15)9-7/h2-4,10-12H,1H2,(H2,8,9,13,15)/t2-,3+,4?,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102883
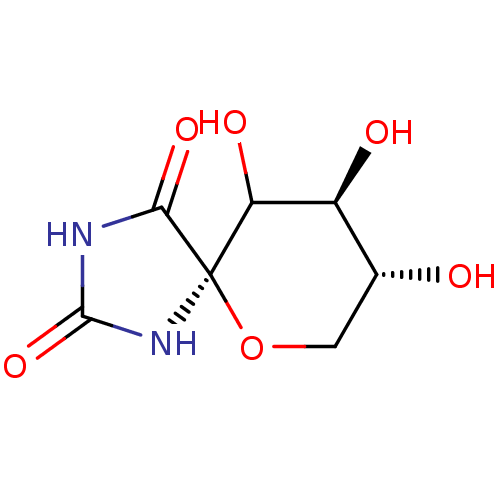
(8,9,10-Trihydroxy-6-oxa-1,3-diaza-spiro[4.5]decane...)Show InChI InChI=1S/C7H10N2O6/c10-2-1-15-7(4(12)3(2)11)5(13)8-6(14)9-7/h2-4,10-12H,1H2,(H2,8,9,13,14)/t2-,3+,4?,7-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, brain form
(Homo sapiens (Human)) | BDBM50102886
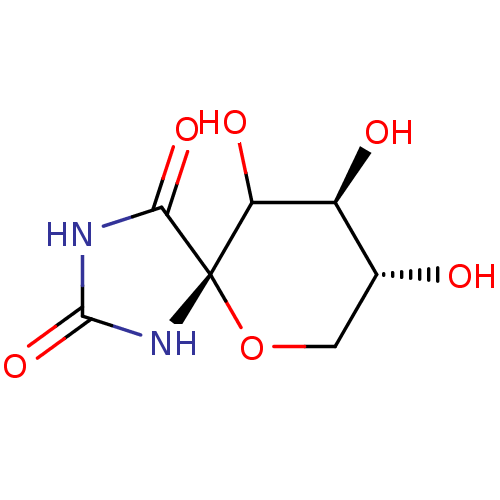
(8,9,10-Trihydroxy-6-oxa-1,3-diaza-spiro[4.5]decane...)Show InChI InChI=1S/C7H10N2O6/c10-2-1-15-7(4(12)3(2)11)5(13)8-6(14)9-7/h2-4,10-12H,1H2,(H2,8,9,13,14)/t2-,3+,4?,7+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.15E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Debrecen
Curated by ChEMBL
| Assay Description
Inhibitory activity against muscle Glycogen Phosphorylase b |
J Med Chem 44: 2843-8 (2001)
BindingDB Entry DOI: 10.7270/Q2NV9JZQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data