

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50103860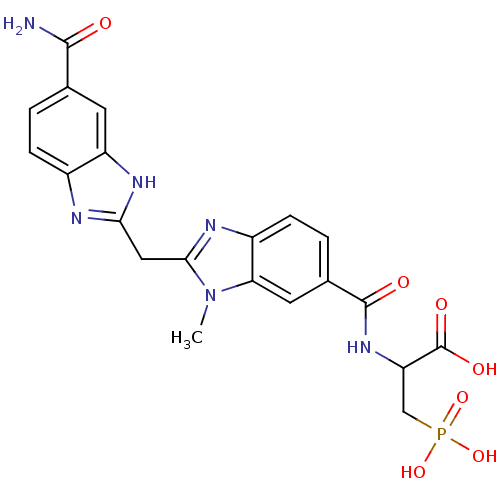 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103862 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103861 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103871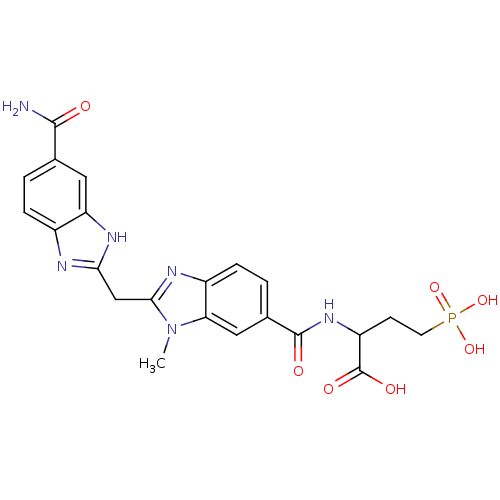 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103854 (2-(3-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103858 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103870 (2-(3-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103855 (2-(3-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103853 (2-(4-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103857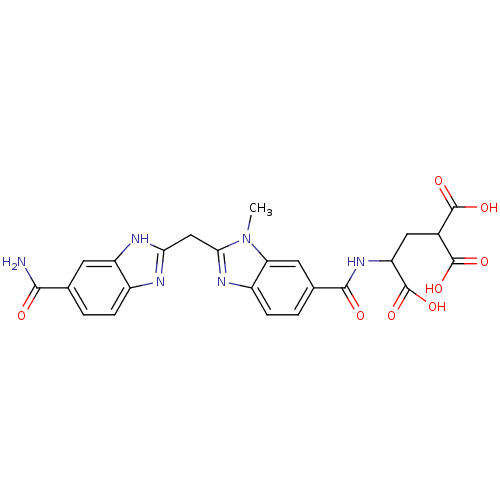 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103873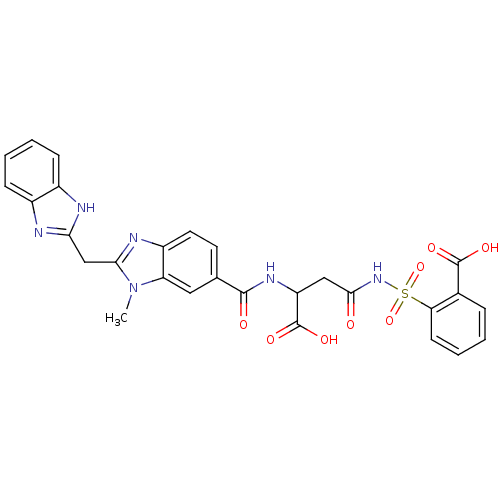 (2-(3-{[2-(1H-Benzoimidazol-2-ylmethyl)-3-methyl-3H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103859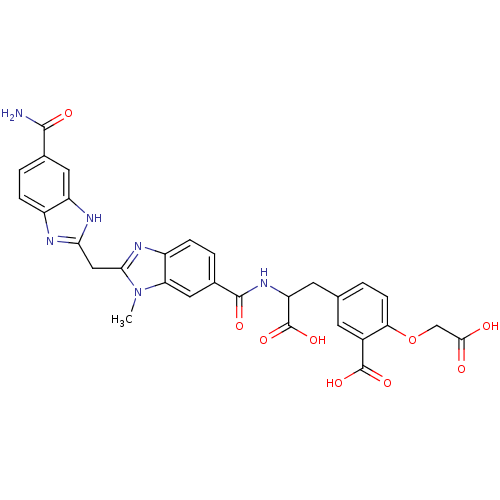 (5-(2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103851 (2-(3-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103868 (5-(2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103863 (4-(Bis-carboxymethyl-carbamoyl)-2-{[2-(5-carbamoyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103856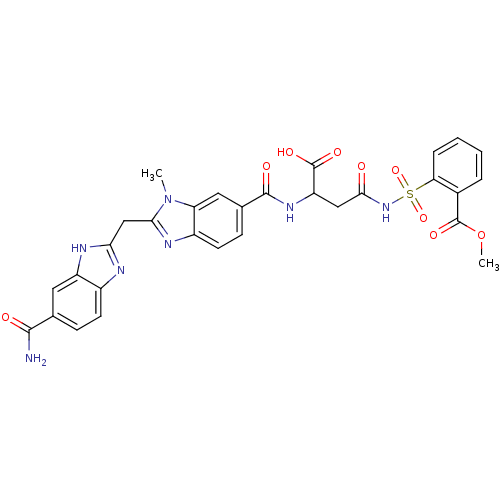 (2-(3-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103850 (4-(2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103864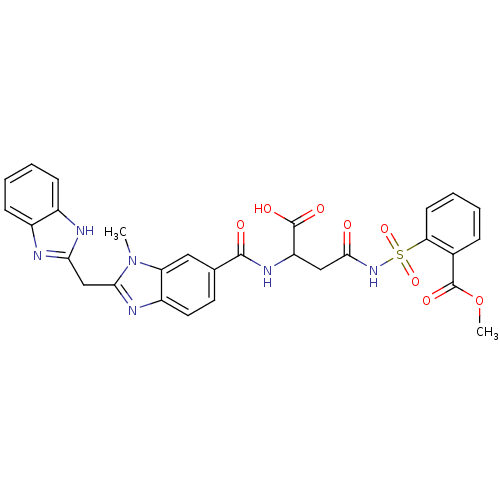 (2-(3-{[2-(1H-Benzoimidazol-2-ylmethyl)-3-methyl-3H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103849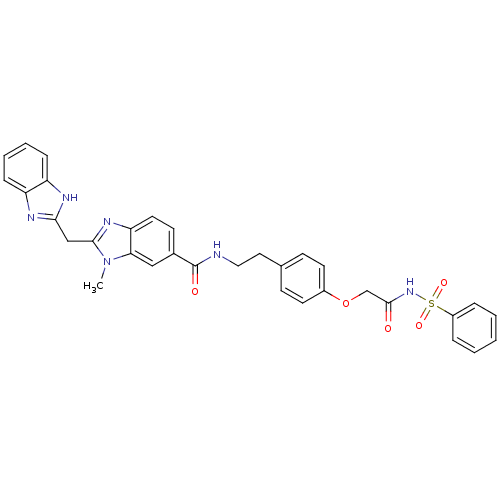 (2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-meth...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103872 (2-(2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103865 (CHEMBL306151 | [4-(2-{[2-(5-Carbamoyl-1H-benzoimid...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103866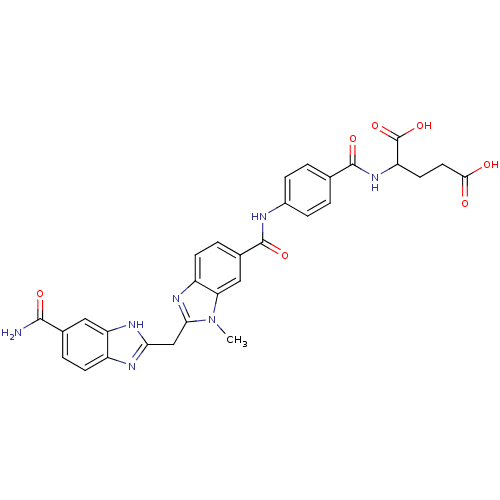 (2-(4-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103867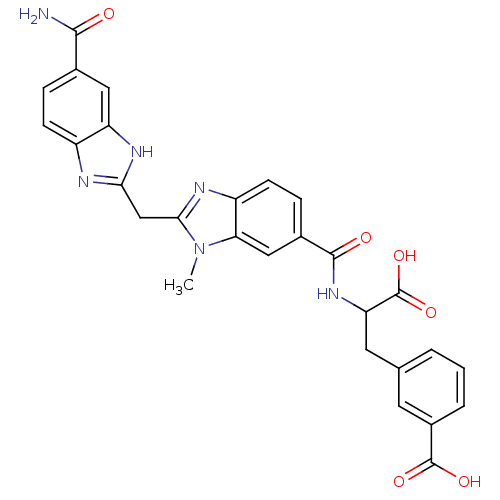 (3-(2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103852 (2-(4-((S)-Benzyloxycarbonyl)-4-{[2-(5-carbamoyl-1H...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103869 (2-((S)-3-Benzyloxycarbonyl-3-{[2-(5-carbamoyl-1H-b...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103874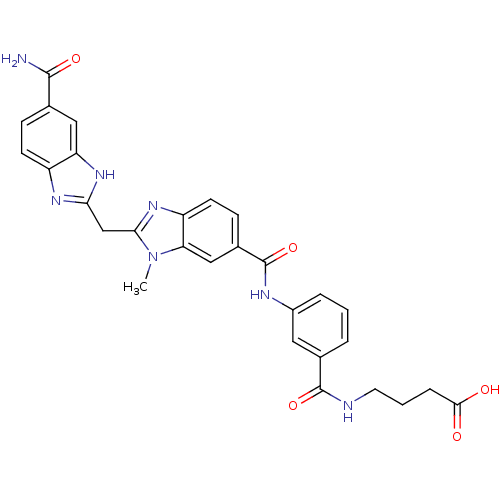 (4-(3-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against hepatitis C virus (HCV) NS3 protease in the presence of Zn2+. | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50103860 (2-{[2-(5-Carbamoyl-1H-benzoimidazol-2-ylmethyl)-3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against hepatitis C virus (HCV) NS3 protease in the absence of Zn2+ | Bioorg Med Chem Lett 11: 2355-9 (2001) BindingDB Entry DOI: 10.7270/Q2N015TQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||