 Found 65 hits of Enzyme Inhibition Constant Data
Found 65 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106064
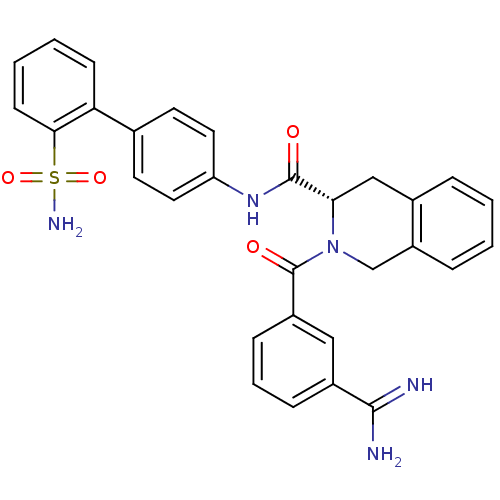
((S)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106056
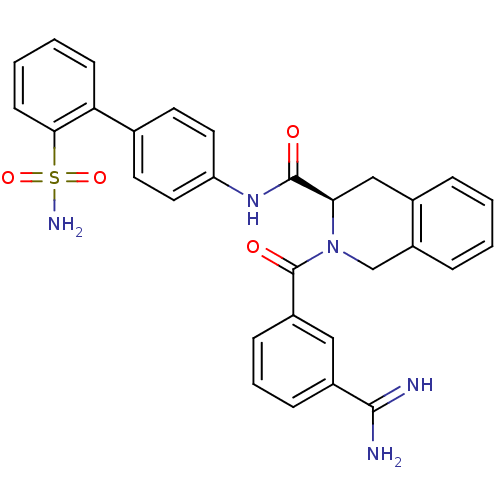
((R)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106058
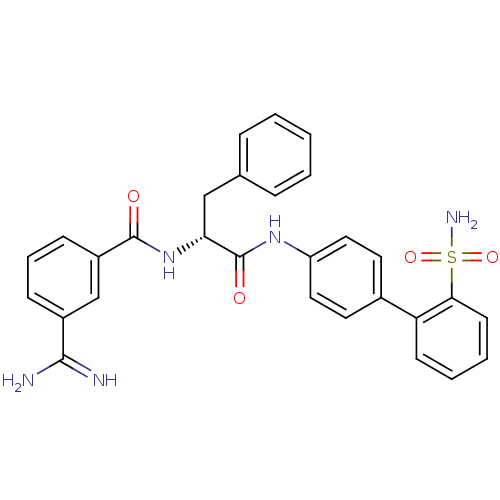
(3-Carbamimidoyl-N-[(R)-2-phenyl-1-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@H](Cc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C29H27N5O4S/c30-27(31)21-9-6-10-22(18-21)28(35)34-25(17-19-7-2-1-3-8-19)29(36)33-23-15-13-20(14-16-23)24-11-4-5-12-26(24)39(32,37)38/h1-16,18,25H,17H2,(H3,30,31)(H,33,36)(H,34,35)(H2,32,37,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106059
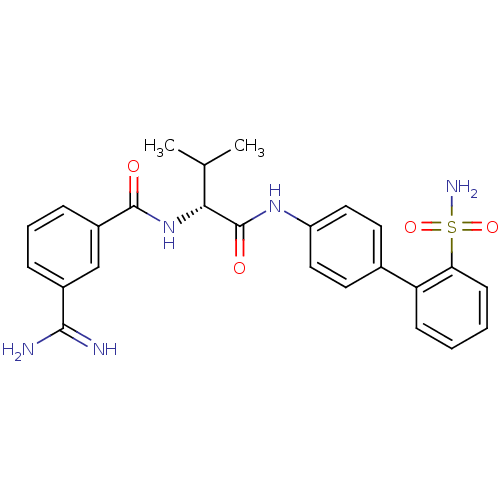
(3-Carbamimidoyl-N-[(R)-2-methyl-1-(2'-sulfamoyl-bi...)Show SMILES CC(C)[C@@H](NC(=O)c1cccc(c1)C(N)=N)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H27N5O4S/c1-15(2)22(30-24(31)18-7-5-6-17(14-18)23(26)27)25(32)29-19-12-10-16(11-13-19)20-8-3-4-9-21(20)35(28,33)34/h3-15,22H,1-2H3,(H3,26,27)(H,29,32)(H,30,31)(H2,28,33,34)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106057
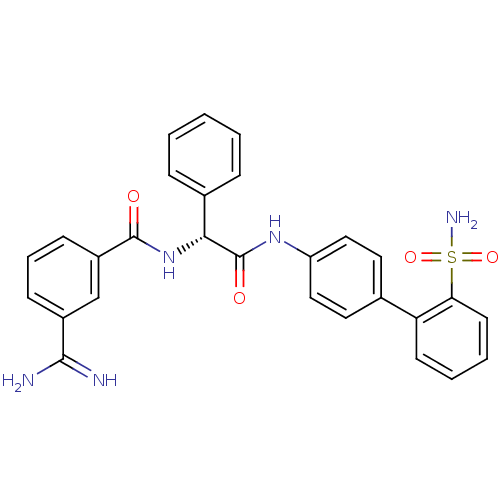
(3-Carbamimidoyl-N-[(R)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106055
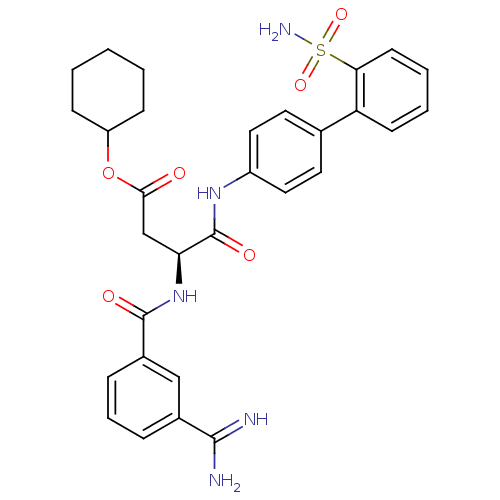
((S)-3-(3-Carbamimidoyl-benzoylamino)-N-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CC(=O)OC1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H33N5O6S/c31-28(32)20-7-6-8-21(17-20)29(37)35-25(18-27(36)41-23-9-2-1-3-10-23)30(38)34-22-15-13-19(14-16-22)24-11-4-5-12-26(24)42(33,39)40/h4-8,11-17,23,25H,1-3,9-10,18H2,(H3,31,32)(H,34,38)(H,35,37)(H2,33,39,40)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106051
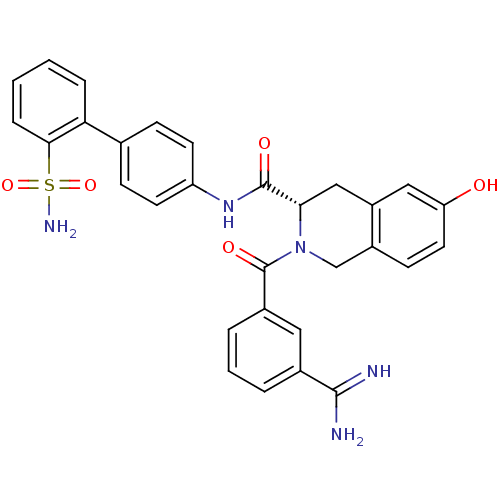
((S)-2-(3-Carbamimidoyl-benzoyl)-6-hydroxy-1,2,3,4-...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccc(O)cc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O5S/c31-28(32)19-4-3-5-20(14-19)30(38)35-17-21-10-13-24(36)15-22(21)16-26(35)29(37)34-23-11-8-18(9-12-23)25-6-1-2-7-27(25)41(33,39)40/h1-15,26,36H,16-17H2,(H3,31,32)(H,34,37)(H2,33,39,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106052

(3-Carbamimidoyl-N-[(S)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 337 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106063

(3-Carbamimidoyl-N-[(S)-cyclohexyl-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C28H31N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h4-6,9-17,19,25H,1-3,7-8H2,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 635 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106062
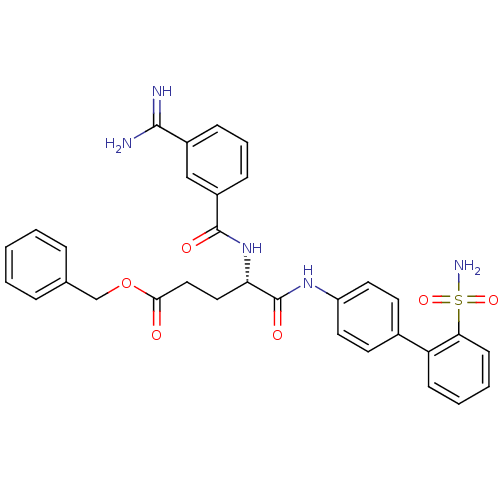
((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(=O)OCc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C32H31N5O6S/c33-30(34)23-9-6-10-24(19-23)31(39)37-27(17-18-29(38)43-20-21-7-2-1-3-8-21)32(40)36-25-15-13-22(14-16-25)26-11-4-5-12-28(26)44(35,41)42/h1-16,19,27H,17-18,20H2,(H3,33,34)(H,36,40)(H,37,39)(H2,35,41,42)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 644 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106060
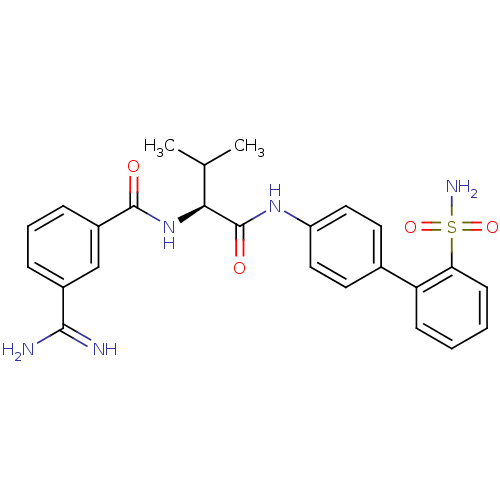
(3-Carbamimidoyl-N-[(S)-2-methyl-1-(2'-sulfamoyl-bi...)Show SMILES CC(C)[C@H](NC(=O)c1cccc(c1)C(N)=N)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H27N5O4S/c1-15(2)22(30-24(31)18-7-5-6-17(14-18)23(26)27)25(32)29-19-12-10-16(11-13-19)20-8-3-4-9-21(20)35(28,33)34/h3-15,22H,1-2H3,(H3,26,27)(H,29,32)(H,30,31)(H2,28,33,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106050
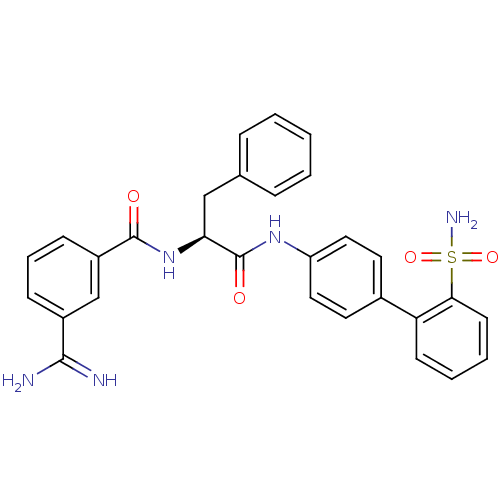
(3-Carbamimidoyl-N-[(S)-2-phenyl-1-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C29H27N5O4S/c30-27(31)21-9-6-10-22(18-21)28(35)34-25(17-19-7-2-1-3-8-19)29(36)33-23-15-13-20(14-16-23)24-11-4-5-12-26(24)39(32,37)38/h1-16,18,25H,17H2,(H3,30,31)(H,33,36)(H,34,35)(H2,32,37,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 762 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106053
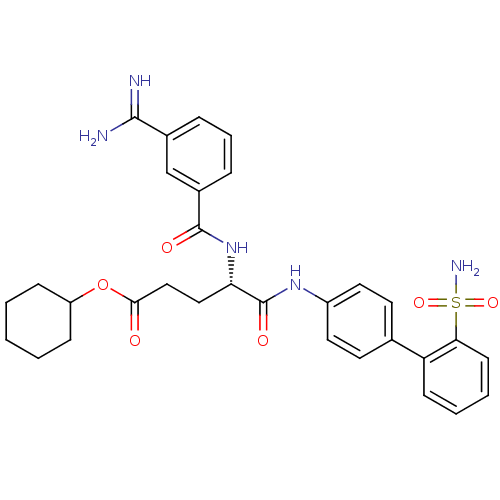
((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(=O)OC1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C31H35N5O6S/c32-29(33)21-7-6-8-22(19-21)30(38)36-26(17-18-28(37)42-24-9-2-1-3-10-24)31(39)35-23-15-13-20(14-16-23)25-11-4-5-12-27(25)43(34,40)41/h4-8,11-16,19,24,26H,1-3,9-10,17-18H2,(H3,32,33)(H,35,39)(H,36,38)(H2,34,40,41)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 842 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106069
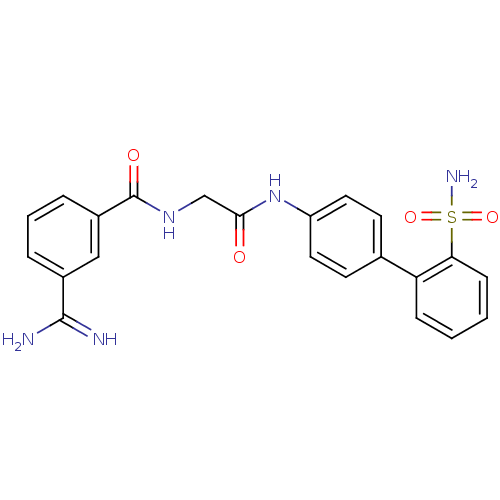
(3-Carbamimidoyl-N-[(2'-sulfamoyl-biphenyl-4-ylcarb...)Show SMILES NC(=N)c1cccc(c1)C(=O)NCC(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H21N5O4S/c23-21(24)15-4-3-5-16(12-15)22(29)26-13-20(28)27-17-10-8-14(9-11-17)18-6-1-2-7-19(18)32(25,30)31/h1-12H,13H2,(H3,23,24)(H,26,29)(H,27,28)(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 851 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106051

((S)-2-(3-Carbamimidoyl-benzoyl)-6-hydroxy-1,2,3,4-...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccc(O)cc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O5S/c31-28(32)19-4-3-5-20(14-19)30(38)35-17-21-10-13-24(36)15-22(21)16-26(35)29(37)34-23-11-8-18(9-12-23)25-6-1-2-7-27(25)41(33,39)40/h1-15,26,36H,16-17H2,(H3,31,32)(H,34,37)(H2,33,39,40)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 888 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106067
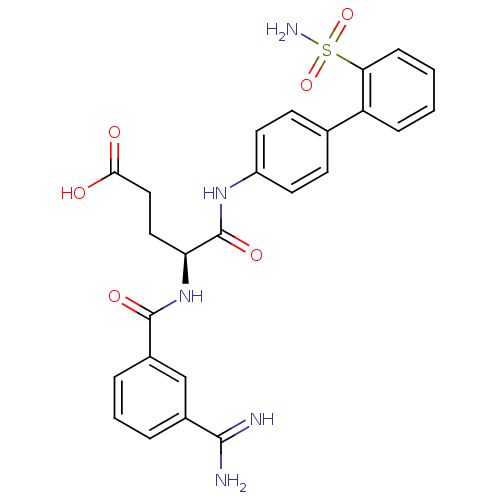
((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H25N5O6S/c26-23(27)16-4-3-5-17(14-16)24(33)30-20(12-13-22(31)32)25(34)29-18-10-8-15(9-11-18)19-6-1-2-7-21(19)37(28,35)36/h1-11,14,20H,12-13H2,(H3,26,27)(H,29,34)(H,30,33)(H,31,32)(H2,28,35,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106057

(3-Carbamimidoyl-N-[(R)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106049
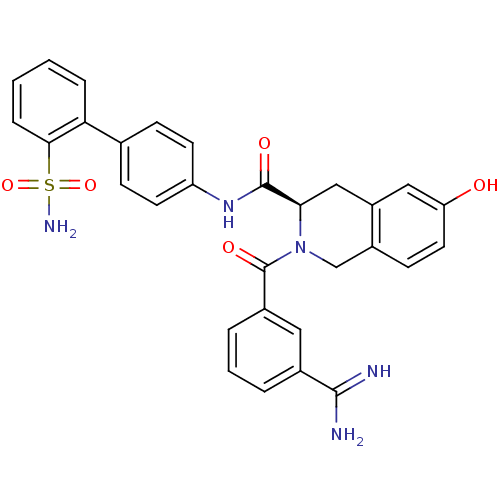
((R)-2-(3-Carbamimidoyl-benzoyl)-6-hydroxy-1,2,3,4-...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccc(O)cc2C[C@@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O5S/c31-28(32)19-4-3-5-20(14-19)30(38)35-17-21-10-13-24(36)15-22(21)16-26(35)29(37)34-23-11-8-18(9-12-23)25-6-1-2-7-27(25)41(33,39)40/h1-15,26,36H,16-17H2,(H3,31,32)(H,34,37)(H2,33,39,40)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106064

((S)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106068
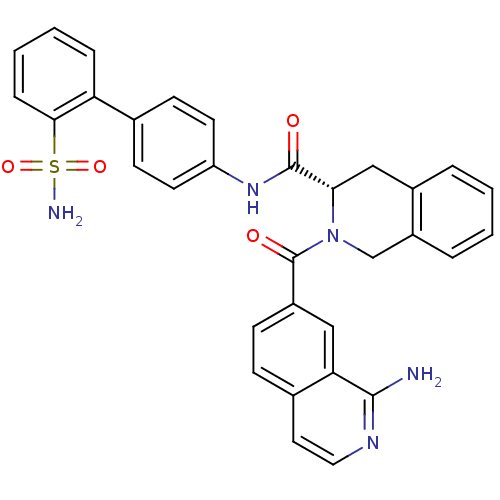
((S)-2-(1-Amino-isoquinoline-7-carbonyl)-1,2,3,4-te...)Show SMILES Nc1nccc2ccc(cc12)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C32H27N5O4S/c33-30-27-17-23(10-9-21(27)15-16-35-30)32(39)37-19-24-6-2-1-5-22(24)18-28(37)31(38)36-25-13-11-20(12-14-25)26-7-3-4-8-29(26)42(34,40)41/h1-17,28H,18-19H2,(H2,33,35)(H,36,38)(H2,34,40,41)/t28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106066
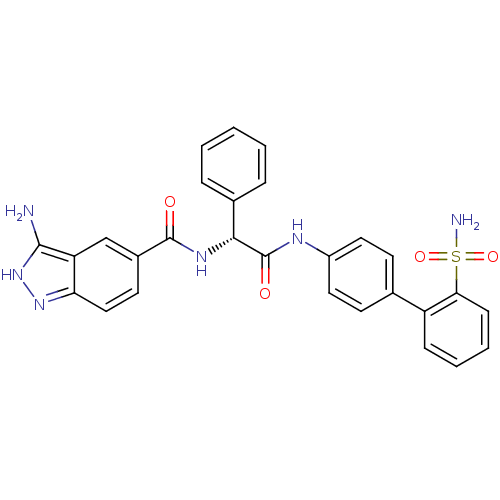
(3-Amino-1H-indazole-5-carboxylic acid [(R)-phenyl-...)Show SMILES Nc1[nH]nc2ccc(cc12)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H24N6O4S/c29-26-22-16-19(12-15-23(22)33-34-26)27(35)32-25(18-6-2-1-3-7-18)28(36)31-20-13-10-17(11-14-20)21-8-4-5-9-24(21)39(30,37)38/h1-16,25H,(H,31,36)(H,32,35)(H3,29,33,34)(H2,30,37,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106056

((R)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106055

((S)-3-(3-Carbamimidoyl-benzoylamino)-N-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CC(=O)OC1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H33N5O6S/c31-28(32)20-7-6-8-21(17-20)29(37)35-25(18-27(36)41-23-9-2-1-3-10-23)30(38)34-22-15-13-19(14-16-22)24-11-4-5-12-26(24)42(33,39)40/h4-8,11-17,23,25H,1-3,9-10,18H2,(H3,31,32)(H,34,38)(H,35,37)(H2,33,39,40)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106052

(3-Carbamimidoyl-N-[(S)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106062

((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(=O)OCc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C32H31N5O6S/c33-30(34)23-9-6-10-24(19-23)31(39)37-27(17-18-29(38)43-20-21-7-2-1-3-8-21)32(40)36-25-15-13-22(14-16-25)26-11-4-5-12-28(26)44(35,41)42/h1-16,19,27H,17-18,20H2,(H3,33,34)(H,36,40)(H,37,39)(H2,35,41,42)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50106057

(3-Carbamimidoyl-N-[(R)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against activated protein C (APC) |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106063

(3-Carbamimidoyl-N-[(S)-cyclohexyl-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C28H31N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h4-6,9-17,19,25H,1-3,7-8H2,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106061
((S)-1-(3-Carbamimidoyl-benzoyl)-pyrrolidine-2-carb...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H25N5O4S/c26-23(27)17-5-3-6-18(15-17)25(32)30-14-4-8-21(30)24(31)29-19-12-10-16(11-13-19)20-7-1-2-9-22(20)35(28,33)34/h1-3,5-7,9-13,15,21H,4,8,14H2,(H3,26,27)(H,29,31)(H2,28,33,34)/t21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106053

((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(=O)OC1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C31H35N5O6S/c32-29(33)21-7-6-8-22(19-21)30(38)36-26(17-18-28(37)42-24-9-2-1-3-10-24)31(39)35-23-15-13-20(14-16-23)25-11-4-5-12-27(25)43(34,40)41/h4-8,11-16,19,24,26H,1-3,9-10,17-18H2,(H3,32,33)(H,35,39)(H,36,38)(H2,34,40,41)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106061

((S)-1-(3-Carbamimidoyl-benzoyl)-pyrrolidine-2-carb...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H25N5O4S/c26-23(27)17-5-3-6-18(15-17)25(32)30-14-4-8-21(30)24(31)29-19-12-10-16(11-13-19)20-7-1-2-9-22(20)35(28,33)34/h1-3,5-7,9-13,15,21H,4,8,14H2,(H3,26,27)(H,29,31)(H2,28,33,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106069

(3-Carbamimidoyl-N-[(2'-sulfamoyl-biphenyl-4-ylcarb...)Show SMILES NC(=N)c1cccc(c1)C(=O)NCC(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H21N5O4S/c23-21(24)15-4-3-5-16(12-15)22(29)26-13-20(28)27-17-10-8-14(9-11-17)18-6-1-2-7-19(18)32(25,30)31/h1-12H,13H2,(H3,23,24)(H,26,29)(H,27,28)(H2,25,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106054

(3-Amino-benzo[d]isoxazole-5-carboxylic acid [(R)-p...)Show SMILES Nc1noc2ccc(cc12)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H23N5O5S/c29-26-22-16-19(12-15-23(22)38-33-26)27(34)32-25(18-6-2-1-3-7-18)28(35)31-20-13-10-17(11-14-20)21-8-4-5-9-24(21)39(30,36)37/h1-16,25H,(H2,29,33)(H,31,35)(H,32,34)(H2,30,36,37)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Coagulation factor X was determined |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106059

(3-Carbamimidoyl-N-[(R)-2-methyl-1-(2'-sulfamoyl-bi...)Show SMILES CC(C)[C@@H](NC(=O)c1cccc(c1)C(N)=N)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H27N5O4S/c1-15(2)22(30-24(31)18-7-5-6-17(14-18)23(26)27)25(32)29-19-12-10-16(11-13-19)20-8-3-4-9-21(20)35(28,33)34/h3-15,22H,1-2H3,(H3,26,27)(H,29,32)(H,30,31)(H2,28,33,34)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106052

(3-Carbamimidoyl-N-[(S)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106059

(3-Carbamimidoyl-N-[(R)-2-methyl-1-(2'-sulfamoyl-bi...)Show SMILES CC(C)[C@@H](NC(=O)c1cccc(c1)C(N)=N)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H27N5O4S/c1-15(2)22(30-24(31)18-7-5-6-17(14-18)23(26)27)25(32)29-19-12-10-16(11-13-19)20-8-3-4-9-21(20)35(28,33)34/h3-15,22H,1-2H3,(H3,26,27)(H,29,32)(H,30,31)(H2,28,33,34)/t22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106056

((R)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106049

((R)-2-(3-Carbamimidoyl-benzoyl)-6-hydroxy-1,2,3,4-...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccc(O)cc2C[C@@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O5S/c31-28(32)19-4-3-5-20(14-19)30(38)35-17-21-10-13-24(36)15-22(21)16-26(35)29(37)34-23-11-8-18(9-12-23)25-6-1-2-7-27(25)41(33,39)40/h1-15,26,36H,16-17H2,(H3,31,32)(H,34,37)(H2,33,39,40)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106060

(3-Carbamimidoyl-N-[(S)-2-methyl-1-(2'-sulfamoyl-bi...)Show SMILES CC(C)[C@H](NC(=O)c1cccc(c1)C(N)=N)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H27N5O4S/c1-15(2)22(30-24(31)18-7-5-6-17(14-18)23(26)27)25(32)29-19-12-10-16(11-13-19)20-8-3-4-9-21(20)35(28,33)34/h3-15,22H,1-2H3,(H3,26,27)(H,29,32)(H,30,31)(H2,28,33,34)/t22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106063

(3-Carbamimidoyl-N-[(S)-cyclohexyl-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C28H31N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h4-6,9-17,19,25H,1-3,7-8H2,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106058

(3-Carbamimidoyl-N-[(R)-2-phenyl-1-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@H](Cc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C29H27N5O4S/c30-27(31)21-9-6-10-22(18-21)28(35)34-25(17-19-7-2-1-3-8-19)29(36)33-23-15-13-20(14-16-23)24-11-4-5-12-26(24)39(32,37)38/h1-16,18,25H,17H2,(H3,30,31)(H,33,36)(H,34,35)(H2,32,37,38)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106060

(3-Carbamimidoyl-N-[(S)-2-methyl-1-(2'-sulfamoyl-bi...)Show SMILES CC(C)[C@H](NC(=O)c1cccc(c1)C(N)=N)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H27N5O4S/c1-15(2)22(30-24(31)18-7-5-6-17(14-18)23(26)27)25(32)29-19-12-10-16(11-13-19)20-8-3-4-9-21(20)35(28,33)34/h3-15,22H,1-2H3,(H3,26,27)(H,29,32)(H,30,31)(H2,28,33,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106065

(3-Amino-1H-indazole-5-carboxylic acid [(S)-phenyl-...)Show SMILES Nc1[nH]nc2ccc(cc12)C(=O)N[C@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H24N6O4S/c29-26-22-16-19(12-15-23(22)33-34-26)27(35)32-25(18-6-2-1-3-7-18)28(36)31-20-13-10-17(11-14-20)21-8-4-5-9-24(21)39(30,37)38/h1-16,25H,(H,31,36)(H,32,35)(H3,29,33,34)(H2,30,37,38)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106067

((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H25N5O6S/c26-23(27)16-4-3-5-17(14-16)24(33)30-20(12-13-22(31)32)25(34)29-18-10-8-15(9-11-18)19-6-1-2-7-21(19)37(28,35)36/h1-11,14,20H,12-13H2,(H3,26,27)(H,29,34)(H,30,33)(H,31,32)(H2,28,35,36)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106050

(3-Carbamimidoyl-N-[(S)-2-phenyl-1-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C29H27N5O4S/c30-27(31)21-9-6-10-22(18-21)28(35)34-25(17-19-7-2-1-3-8-19)29(36)33-23-15-13-20(14-16-23)24-11-4-5-12-26(24)39(32,37)38/h1-16,18,25H,17H2,(H3,30,31)(H,33,36)(H,34,35)(H2,32,37,38)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106061

((S)-1-(3-Carbamimidoyl-benzoyl)-pyrrolidine-2-carb...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1CCC[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H25N5O4S/c26-23(27)17-5-3-6-18(15-17)25(32)30-14-4-8-21(30)24(31)29-19-12-10-16(11-13-19)20-7-1-2-9-22(20)35(28,33)34/h1-3,5-7,9-13,15,21H,4,8,14H2,(H3,26,27)(H,29,31)(H2,28,33,34)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106057

(3-Carbamimidoyl-N-[(R)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106067

((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(O)=O)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C25H25N5O6S/c26-23(27)16-4-3-5-17(14-16)24(33)30-20(12-13-22(31)32)25(34)29-18-10-8-15(9-11-18)19-6-1-2-7-21(19)37(28,35)36/h1-11,14,20H,12-13H2,(H3,26,27)(H,29,34)(H,30,33)(H,31,32)(H2,28,35,36)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106066

(3-Amino-1H-indazole-5-carboxylic acid [(R)-phenyl-...)Show SMILES Nc1[nH]nc2ccc(cc12)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H24N6O4S/c29-26-22-16-19(12-15-23(22)33-34-26)27(35)32-25(18-6-2-1-3-7-18)28(36)31-20-13-10-17(11-14-20)21-8-4-5-9-24(21)39(30,37)38/h1-16,25H,(H,31,36)(H,32,35)(H3,29,33,34)(H2,30,37,38)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106051

((S)-2-(3-Carbamimidoyl-benzoyl)-6-hydroxy-1,2,3,4-...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccc(O)cc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O5S/c31-28(32)19-4-3-5-20(14-19)30(38)35-17-21-10-13-24(36)15-22(21)16-26(35)29(37)34-23-11-8-18(9-12-23)25-6-1-2-7-27(25)41(33,39)40/h1-15,26,36H,16-17H2,(H3,31,32)(H,34,37)(H2,33,39,40)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106049

((R)-2-(3-Carbamimidoyl-benzoyl)-6-hydroxy-1,2,3,4-...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccc(O)cc2C[C@@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O5S/c31-28(32)19-4-3-5-20(14-19)30(38)35-17-21-10-13-24(36)15-22(21)16-26(35)29(37)34-23-11-8-18(9-12-23)25-6-1-2-7-27(25)41(33,39)40/h1-15,26,36H,16-17H2,(H3,31,32)(H,34,37)(H2,33,39,40)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106054

(3-Amino-benzo[d]isoxazole-5-carboxylic acid [(R)-p...)Show SMILES Nc1noc2ccc(cc12)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H23N5O5S/c29-26-22-16-19(12-15-23(22)38-33-26)27(34)32-25(18-6-2-1-3-7-18)28(35)31-20-13-10-17(11-14-20)21-8-4-5-9-24(21)39(30,36)37/h1-16,25H,(H2,29,33)(H,31,35)(H,32,34)(H2,30,36,37)/t25-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106058

(3-Carbamimidoyl-N-[(R)-2-phenyl-1-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@H](Cc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C29H27N5O4S/c30-27(31)21-9-6-10-22(18-21)28(35)34-25(17-19-7-2-1-3-8-19)29(36)33-23-15-13-20(14-16-23)24-11-4-5-12-26(24)39(32,37)38/h1-16,18,25H,17H2,(H3,30,31)(H,33,36)(H,34,35)(H2,32,37,38)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106069

(3-Carbamimidoyl-N-[(2'-sulfamoyl-biphenyl-4-ylcarb...)Show SMILES NC(=N)c1cccc(c1)C(=O)NCC(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C22H21N5O4S/c23-21(24)15-4-3-5-16(12-15)22(29)26-13-20(28)27-17-10-8-14(9-11-17)18-6-1-2-7-19(18)32(25,30)31/h1-12H,13H2,(H3,23,24)(H,26,29)(H,27,28)(H2,25,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106068

((S)-2-(1-Amino-isoquinoline-7-carbonyl)-1,2,3,4-te...)Show SMILES Nc1nccc2ccc(cc12)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C32H27N5O4S/c33-30-27-17-23(10-9-21(27)15-16-35-30)32(39)37-19-24-6-2-1-5-22(24)18-28(37)31(38)36-25-13-11-20(12-14-25)26-7-3-4-8-29(26)42(34,40)41/h1-17,28H,18-19H2,(H2,33,35)(H,36,38)(H2,34,40,41)/t28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106062

((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(=O)OCc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C32H31N5O6S/c33-30(34)23-9-6-10-24(19-23)31(39)37-27(17-18-29(38)43-20-21-7-2-1-3-8-21)32(40)36-25-15-13-22(14-16-25)26-11-4-5-12-28(26)44(35,41)42/h1-16,19,27H,17-18,20H2,(H3,33,34)(H,36,40)(H,37,39)(H2,35,41,42)/t27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50106050

(3-Carbamimidoyl-N-[(S)-2-phenyl-1-(2'-sulfamoyl-bi...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](Cc1ccccc1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C29H27N5O4S/c30-27(31)21-9-6-10-22(18-21)28(35)34-25(17-19-7-2-1-3-8-19)29(36)33-23-15-13-20(14-16-23)24-11-4-5-12-26(24)39(32,37)38/h1-16,18,25H,17H2,(H3,30,31)(H,33,36)(H,34,35)(H2,32,37,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human trypsin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106055

((S)-3-(3-Carbamimidoyl-benzoylamino)-N-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CC(=O)OC1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H33N5O6S/c31-28(32)20-7-6-8-21(17-20)29(37)35-25(18-27(36)41-23-9-2-1-3-10-23)30(38)34-22-15-13-19(14-16-22)24-11-4-5-12-26(24)42(33,39)40/h4-8,11-17,23,25H,1-3,9-10,18H2,(H3,31,32)(H,34,38)(H,35,37)(H2,33,39,40)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106053

((S)-4-(3-Carbamimidoyl-benzoylamino)-4-(2'-sulfamo...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](CCC(=O)OC1CCCCC1)C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C31H35N5O6S/c32-29(33)21-7-6-8-22(19-21)30(38)36-26(17-18-28(37)42-24-9-2-1-3-10-24)31(39)35-23-15-13-20(14-16-23)25-11-4-5-12-27(25)43(34,40)41/h4-8,11-16,19,24,26H,1-3,9-10,17-18H2,(H3,32,33)(H,35,39)(H,36,38)(H2,34,40,41)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50106064

((S)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human thrombin. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50106065

(3-Amino-1H-indazole-5-carboxylic acid [(S)-phenyl-...)Show SMILES Nc1[nH]nc2ccc(cc12)C(=O)N[C@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H24N6O4S/c29-26-22-16-19(12-15-23(22)33-34-26)27(35)32-25(18-6-2-1-3-7-18)28(36)31-20-13-10-17(11-14-20)21-8-4-5-9-24(21)39(30,37)38/h1-16,25H,(H,31,36)(H,32,35)(H3,29,33,34)(H2,30,37,38)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human coagulation factor Xa. |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50106057

(3-Carbamimidoyl-N-[(R)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50106064

((S)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against activated protein C (APC) |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50106064

((S)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against plasmin |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106057

(3-Carbamimidoyl-N-[(R)-phenyl-(2'-sulfamoyl-biphen...)Show SMILES NC(=N)c1cccc(c1)C(=O)N[C@@H](C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O)c1ccccc1 Show InChI InChI=1S/C28H25N5O4S/c29-26(30)20-9-6-10-21(17-20)27(34)33-25(19-7-2-1-3-8-19)28(35)32-22-15-13-18(14-16-22)23-11-4-5-12-24(23)38(31,36)37/h1-17,25H,(H3,29,30)(H,32,35)(H,33,34)(H2,31,36,37)/t25-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Tissue plasminogen activator |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50106064

((S)-2-(3-Carbamimidoyl-benzoyl)-1,2,3,4-tetrahydro...)Show SMILES NC(=N)c1cccc(c1)C(=O)N1Cc2ccccc2C[C@H]1C(=O)Nc1ccc(cc1)-c1ccccc1S(N)(=O)=O Show InChI InChI=1S/C30H27N5O4S/c31-28(32)21-8-5-9-22(16-21)30(37)35-18-23-7-2-1-6-20(23)17-26(35)29(36)34-24-14-12-19(13-15-24)25-10-3-4-11-27(25)40(33,38)39/h1-16,26H,17-18H2,(H3,31,32)(H,34,36)(H2,33,38,39)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
COR Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Tissue plasminogen activator |
Bioorg Med Chem Lett 11: 2947-50 (2001)
BindingDB Entry DOI: 10.7270/Q27H1HW6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data