

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106655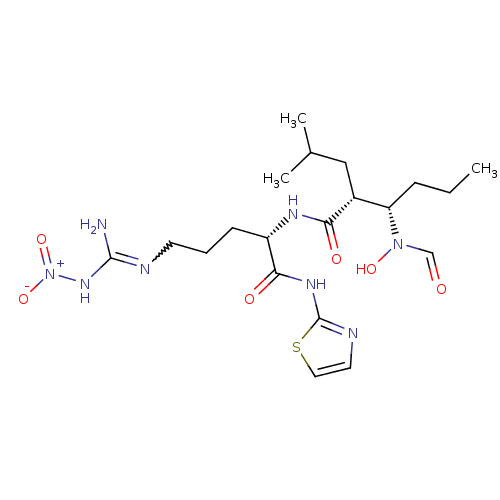 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106652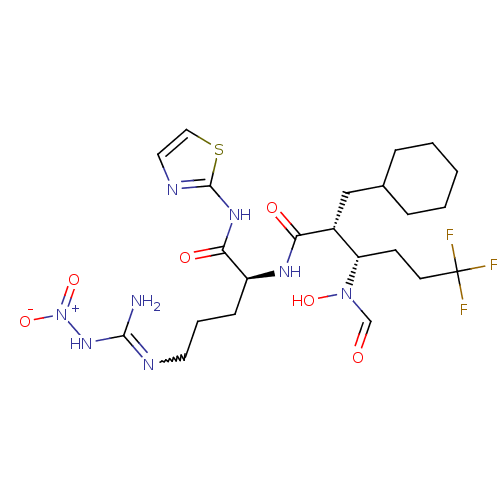 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106653 ((2R,3R)-N-{(1S,2R)-4-Nitroguanyl-2-methyl-1-[(1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106651 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106667 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106658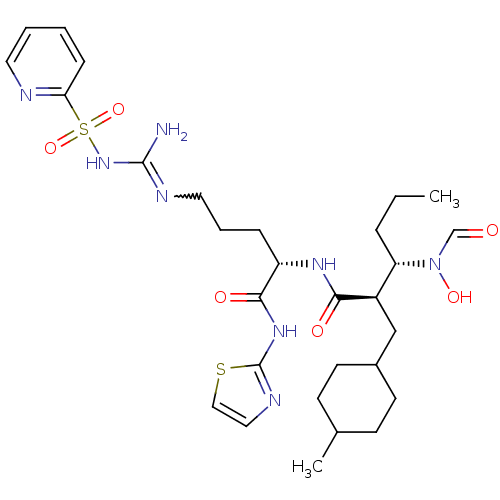 (3-(Formyl-hydroxy-amino)-2-(4-methyl-cyclohexylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106668 ((2R,3R)-N-{(1R)-4-Nitroguanyl-2-[((2R)-2-{(1S)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106656 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106664 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106663 (6,6,6-Trifluoro-3-(formyl-hydroxy-amino)-2-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106659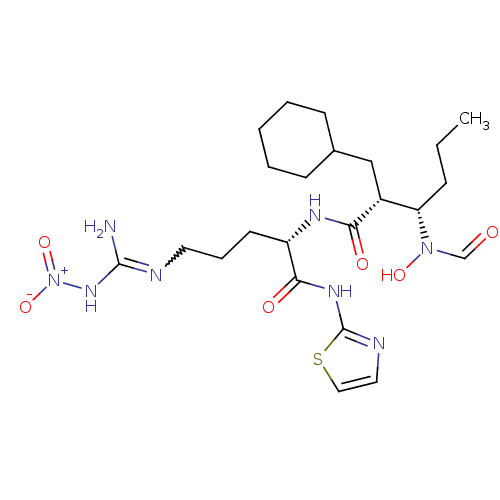 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106657 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106654 (2-[1-(Formyl-hydroxy-amino)-ethyl]-4-methyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106670 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106661 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106671 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106660 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106657 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106654 (2-[1-(Formyl-hydroxy-amino)-ethyl]-4-methyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106653 ((2R,3R)-N-{(1S,2R)-4-Nitroguanyl-2-methyl-1-[(1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106655 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106666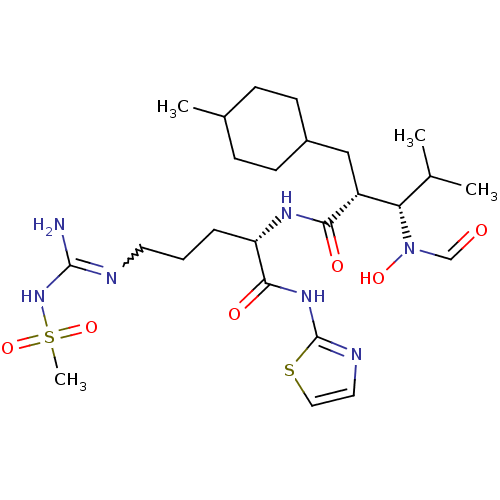 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106662 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106663 (6,6,6-Trifluoro-3-(formyl-hydroxy-amino)-2-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106654 (2-[1-(Formyl-hydroxy-amino)-ethyl]-4-methyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106668 ((2R,3R)-N-{(1R)-4-Nitroguanyl-2-[((2R)-2-{(1S)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106665 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106665 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106669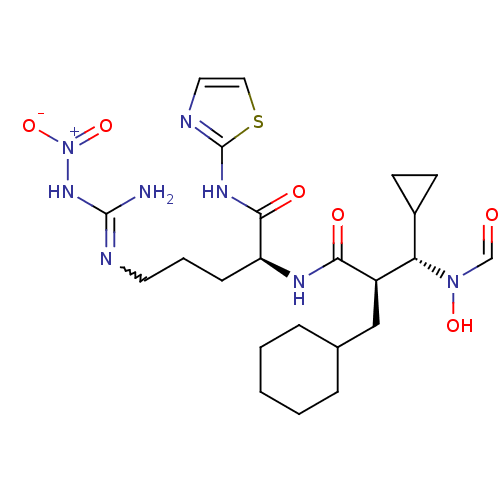 ((2R)-5-Nitroguanyl-2-({(2R,3S)-2-(cyclohexylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106663 (6,6,6-Trifluoro-3-(formyl-hydroxy-amino)-2-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106653 ((2R,3R)-N-{(1S,2R)-4-Nitroguanyl-2-methyl-1-[(1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106660 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106655 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106660 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106655 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106657 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106664 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106668 ((2R,3R)-N-{(1R)-4-Nitroguanyl-2-[((2R)-2-{(1S)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106665 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106667 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106653 ((2R,3R)-N-{(1S,2R)-4-Nitroguanyl-2-methyl-1-[(1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106651 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106652 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106657 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50106660 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using scintillation proximity assay (SPA) | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106656 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106663 (6,6,6-Trifluoro-3-(formyl-hydroxy-amino)-2-isobuty...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106659 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106651 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106665 (3-(Formyl-hydroxy-amino)-2-isobutyl-4-methyl-penta...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106662 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106667 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106664 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106661 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106667 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106664 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106666 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106662 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106656 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106659 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106652 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106662 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106670 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106666 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106656 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106658 (3-(Formyl-hydroxy-amino)-2-(4-methyl-cyclohexylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106658 (3-(Formyl-hydroxy-amino)-2-(4-methyl-cyclohexylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106669 ((2R)-5-Nitroguanyl-2-({(2R,3S)-2-(cyclohexylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106666 (3-(Formyl-hydroxy-amino)-4-methyl-2-(4-methyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106651 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106654 (2-[1-(Formyl-hydroxy-amino)-ethyl]-4-methyl-pentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106670 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106661 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106670 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106659 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106652 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106671 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106668 ((2R,3R)-N-{(1R)-4-Nitroguanyl-2-[((2R)-2-{(1S)-1-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106658 (3-(Formyl-hydroxy-amino)-2-(4-methyl-cyclohexylmet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 282 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106671 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 379 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50106669 ((2R)-5-Nitroguanyl-2-({(2R,3S)-2-(cyclohexylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 602 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-9 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50106669 ((2R)-5-Nitroguanyl-2-({(2R,3S)-2-(cyclohexylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-3 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106661 ((2R,3R)-N-{(1R)-4-Nitroguanyl-1-[(1,3-thiazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50106671 (3-(Formyl-hydroxy-amino)-2-isobutyl-hexanoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition potency was determined by fluorescence-based peptide assay against matrix metalloprotease-1 | J Med Chem 44: 4252-67 (2001) BindingDB Entry DOI: 10.7270/Q24J0DF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||