

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glucagon receptor (Rattus norvegicus) | BDBM50110054 (3-Cyano-4-hydroxy-benzoic acid [1-[4-(4-isopropyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Compound was evaluated in vitro for its inhibitory activity against rat glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110054 (3-Cyano-4-hydroxy-benzoic acid [1-[4-(4-isopropyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110068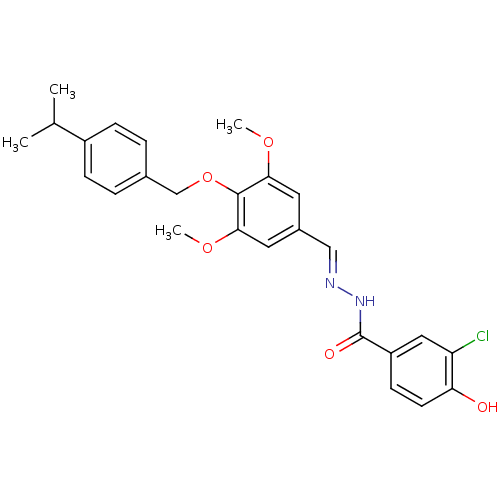 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110056 (2,3-Dichloro-4-hydroxy-benzoic acid [1-[4-(4-isopr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110067 (2,3-Difluoro-4-hydroxy-benzoic acid [1-[4-(4-isopr...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110073 (4-Hydroxy-3-nitro-benzoic acid [1-[4-(4-isopropyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110074 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110066 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-trifluoro...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110055 (3-Chloro-4-hydroxy-benzoic acid [1-(4-isopropyl-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110057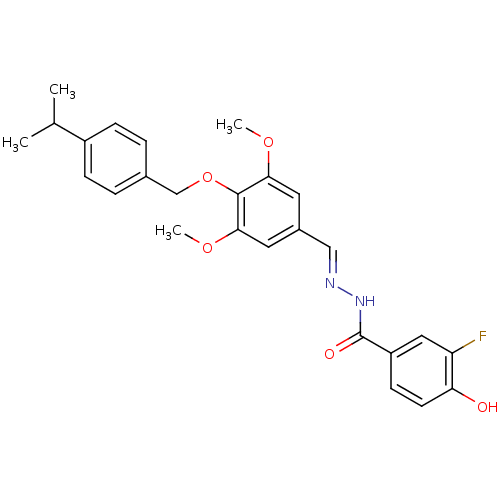 (3-Fluoro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110084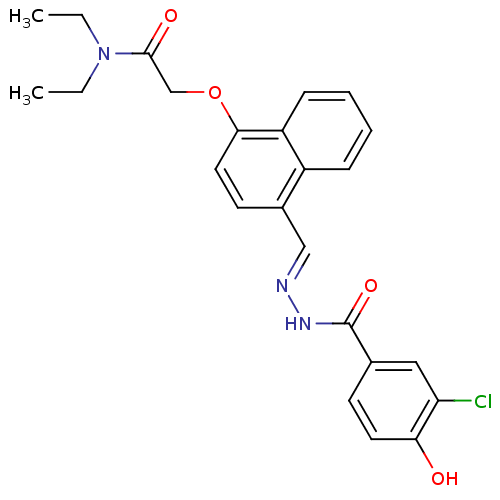 (2-{4-[(3-Chloro-4-hydroxy-benzoyl)-hydrazonomethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110070 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(tetrahydro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110063 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(3,4-difluor...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110053 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(5-tert-buty...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110080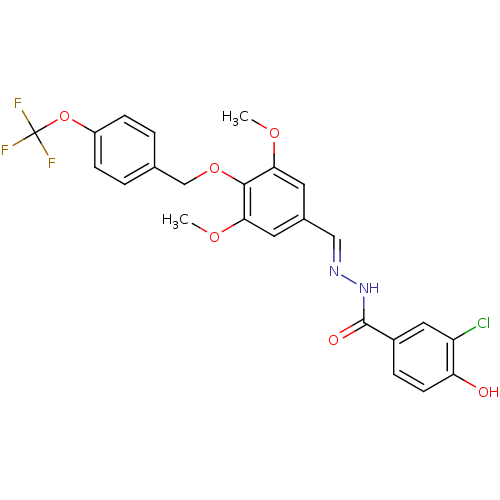 (3-Chloro-4-hydroxy-benzoic acid [1-[3,5-dimethoxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50104096 (3-Chloro-4-hydroxy-benzoic acid (4-hydroxy-naphtha...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110077 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110083 (3-Chloro-4-hydroxy-benzoic acid [1-[1-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110071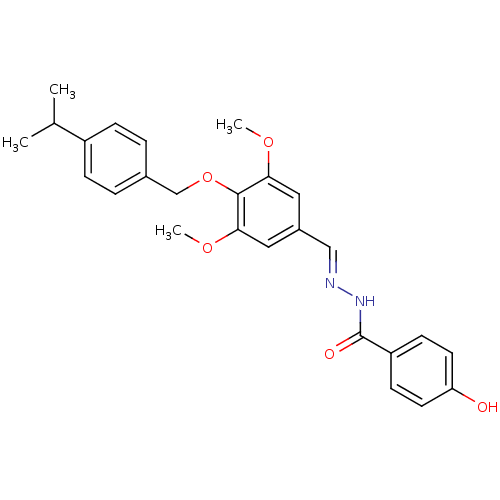 (4-Hydroxy-benzoic acid [1-[4-(4-isopropyl-benzylox...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110081 (CHEMBL161401 | {4-[(3-Chloro-4-hydroxy-benzoyl)-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110085 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110062 (3-Chloro-4-hydroxy-benzoic acid (4-methoxy-naphtha...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110060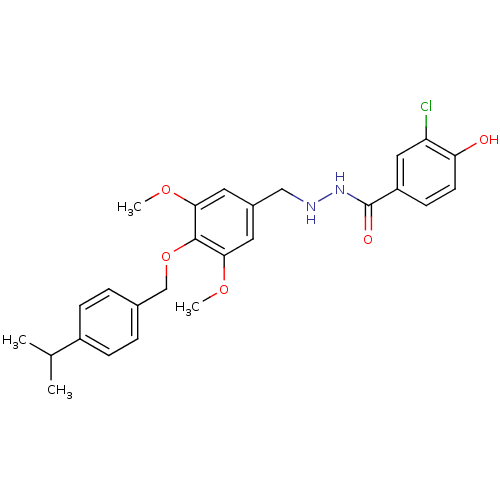 (3-Chloro-4-hydroxy-benzoic acid N'-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110076 (3-Amino-4-hydroxy-benzoic acid [1-[4-(4-isopropyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110065 (2-{4-[(3-Chloro-4-hydroxy-benzoyl)-hydrazonomethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110082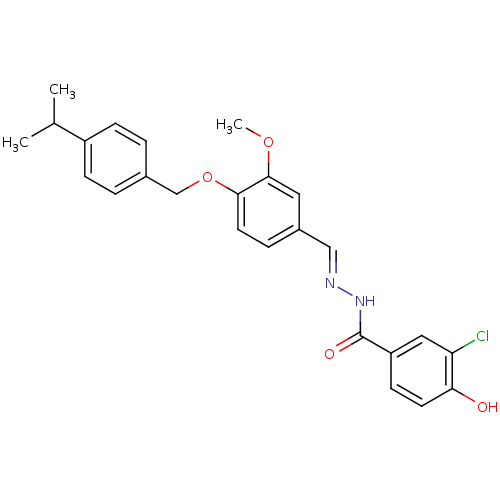 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110078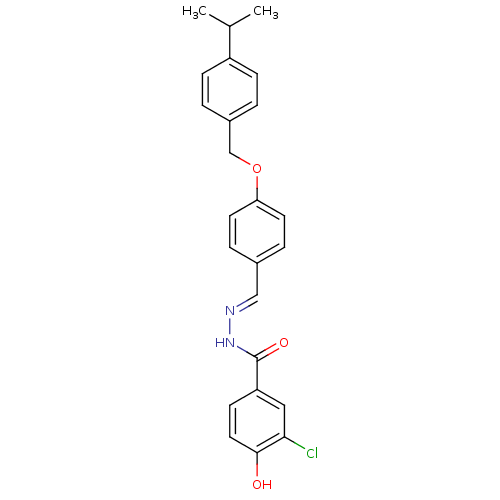 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110069 (3-Chloro-4-hydroxy-N-{2-[4-(4-isopropyl-benzyloxy)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110058 (3,4-Dihydroxy-benzoic acid [1-[4-(4-isopropyl-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110059 (3-Chloro-4-hydroxy-N-{2-[4-(4-isopropyl-benzyloxy)...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110072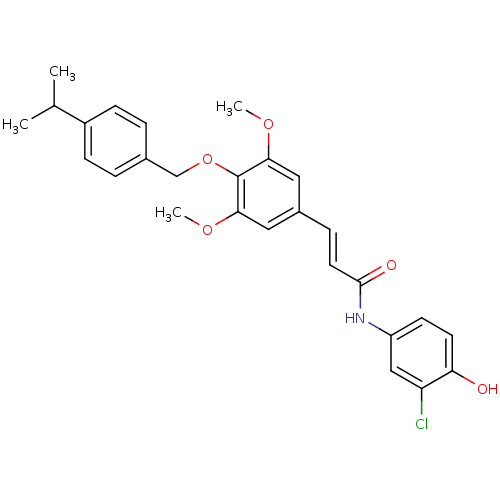 ((E)-N-(3-Chloro-4-hydroxy-phenyl)-3-[4-(4-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110079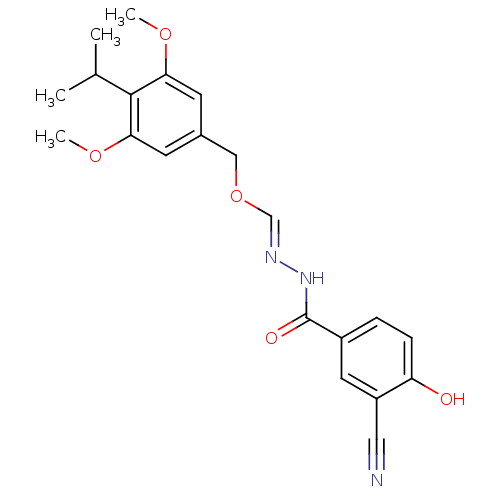 (3-Cyano-4-hydroxy-benzoic acid (4-isopropyl-3,5-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110064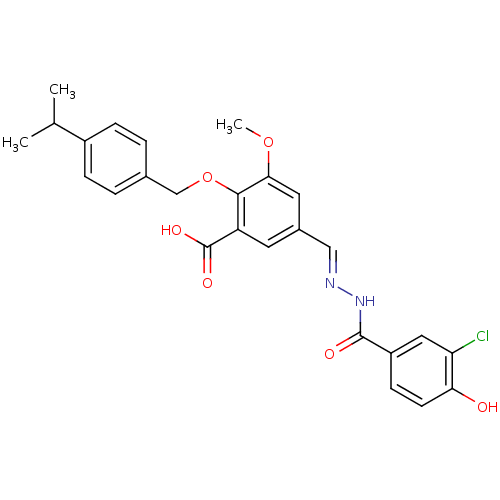 (5-[(3-Chloro-4-hydroxy-benzoyl)-hydrazonomethyl]-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110061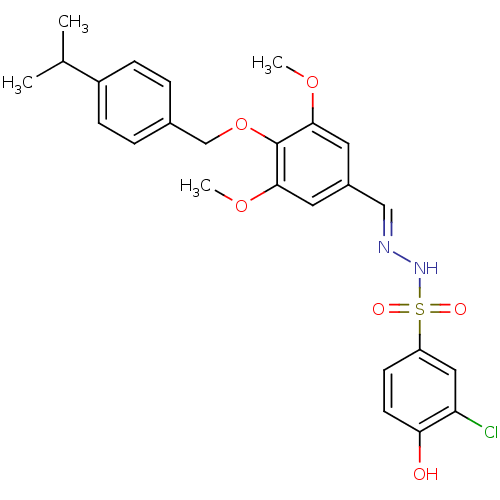 (2-chloro-4-[1-[4-(4-isopropylbenzyloxy)-3,5-dimeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50110075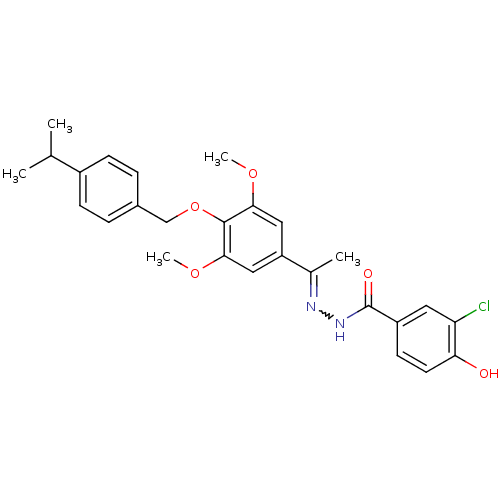 (3-Chloro-4-hydroxy-benzoic acid [1-[4-(4-isopropyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory activity against human glucagon receptor using [127I]-labeled glucagon | Bioorg Med Chem Lett 12: 663-6 (2002) BindingDB Entry DOI: 10.7270/Q2J67G77 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||