

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112833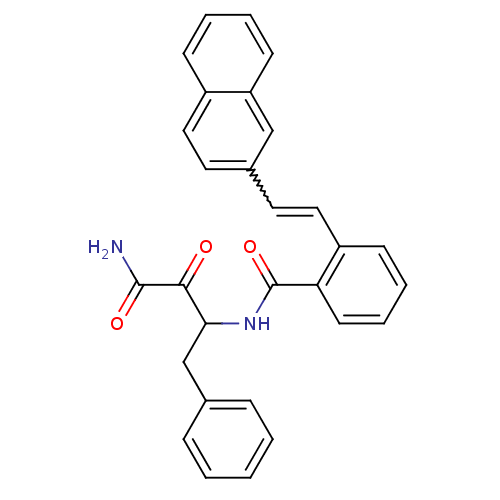 (CHEMBL280587 | N-(1-Benzyl-2-carbamoyl-2-oxo-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50073850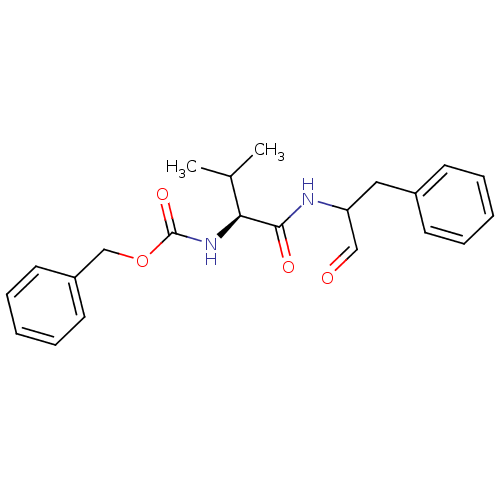 ((S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112845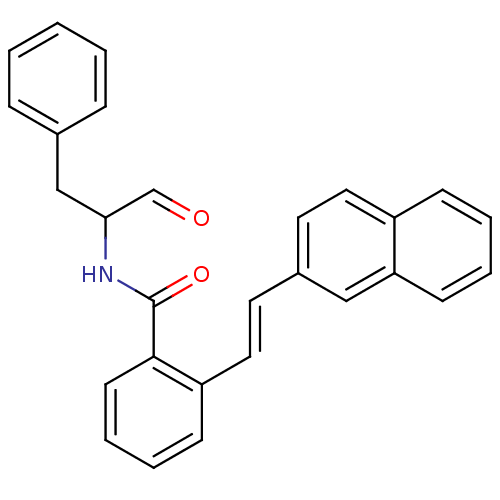 (CHEMBL280654 | N-(1-Benzyl-2-oxo-ethyl)-2-(2-napht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112835 (CHEMBL282979 | N-(1-Benzyl-2-oxo-ethyl)-2-[2-(3,4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112843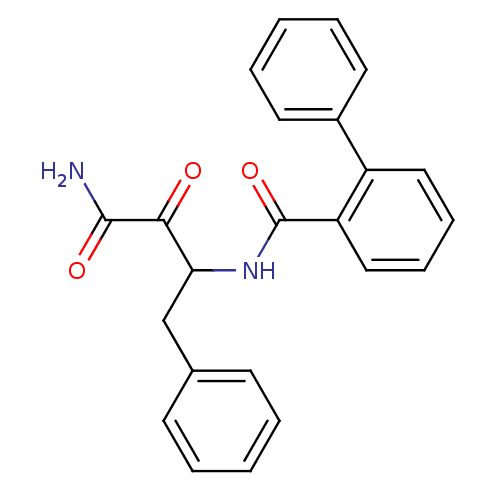 (Biphenyl-2-carboxylic acid (1-benzyl-2-carbamoyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112844 (CHEMBL27036 | N-(1-Benzyl-2-oxo-ethyl)-2-(naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112846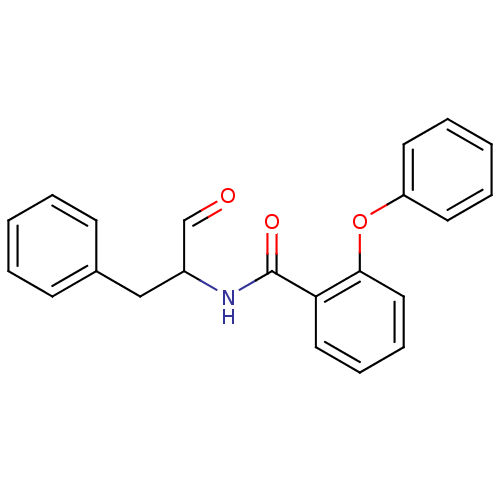 (CHEMBL28203 | N-(1-Benzyl-2-oxo-ethyl)-2-phenoxy-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112832 (CHEMBL441599 | N-(1-Benzyl-2-carbamoyl-2-oxo-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112842 (Biphenyl-2-carboxylic acid (1-benzyl-2-oxo-ethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50112833 (CHEMBL280587 | N-(1-Benzyl-2-carbamoyl-2-oxo-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of cathepsin B | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112839 (CHEMBL26631 | N-(1-Benzyl-2-oxo-ethyl)-2-styryl-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112837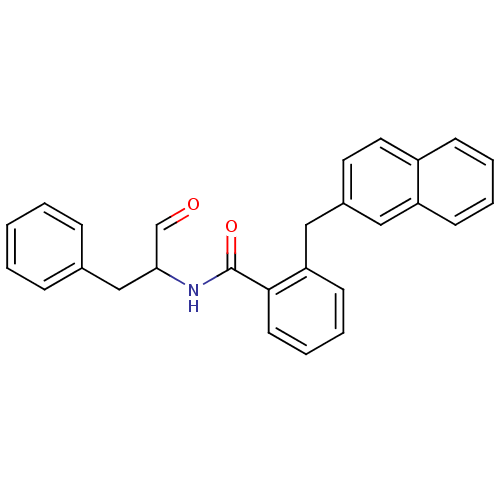 (CHEMBL281731 | N-(1-Benzyl-2-oxo-ethyl)-2-naphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112836 (2-Benzoyl-N-(1-benzyl-2-oxo-ethyl)-benzamide | CHE...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112840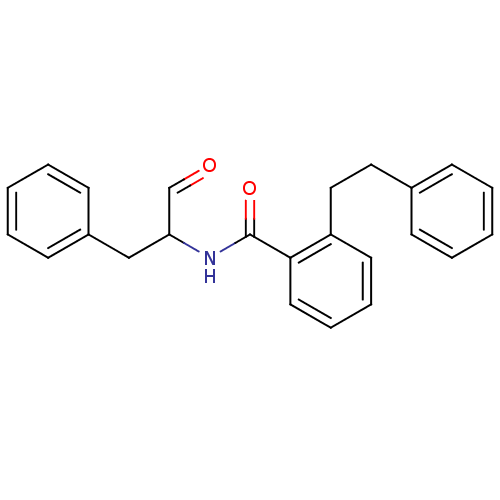 (CHEMBL26870 | N-(1-Benzyl-2-oxo-ethyl)-2-phenethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112847 (2-Benzyl-N-(1-benzyl-2-oxo-ethyl)-benzamide | CHEM...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112841 (CHEMBL280652 | N-(1-Benzyl-2-oxo-ethyl)-2-phenoxym...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112834 (CHEMBL27250 | N-(1-Benzyl-2-oxo-ethyl)-2-phenyleth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Calpain-1 catalytic subunit (Homo sapiens (Human)) | BDBM50112838 (CHEMBL281874 | N-(1-Benzyl-2-oxo-ethyl)-benzamide) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description Inhibition of calpain, using human mu-calpain isolated from erythrocytes and Suc-Leu-Tyr-AMC as the fluorogenic substrate | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50112833 (CHEMBL280587 | N-(1-Benzyl-2-carbamoyl-2-oxo-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH&Co. KG Curated by ChEMBL | Assay Description The compound was evaluated for inhibition of cathepsin L | Bioorg Med Chem Lett 12: 1335-8 (2002) BindingDB Entry DOI: 10.7270/Q2W0958R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||