 Found 380 hits of Enzyme Inhibition Constant Data
Found 380 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50333880
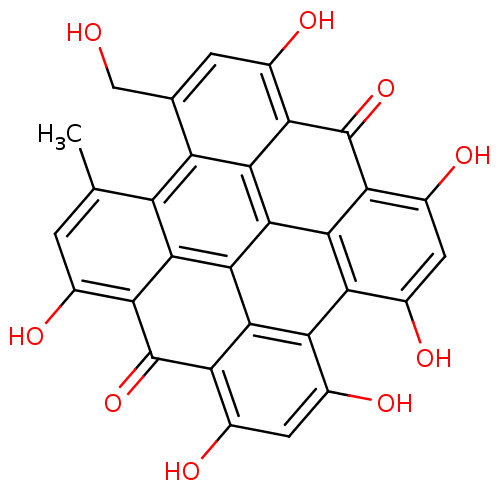
(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 34.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M5
(Homo sapiens (Human)) | BDBM50217942
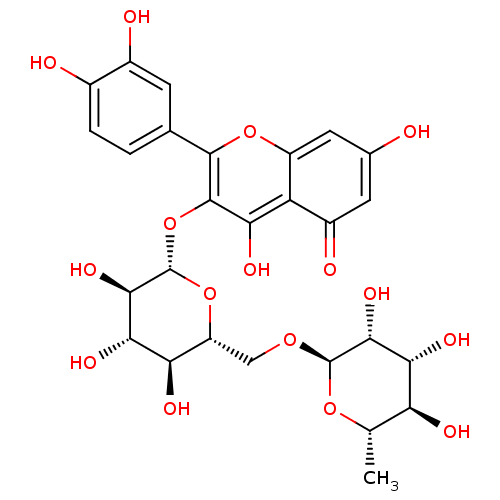
(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 35.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50242259

(CHEMBL516641 | HYPERFORIN)Show SMILES [#6]-[#6](-[#6])-[#6](=O)[C@]12[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-[#6@H](-[#6]\[#6]=[#6](/[#6])-[#6])[C@@]1([#6])[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6]2=O |r,THB:38:37:6.8.14:22.23.29| Show InChI InChI=1S/C35H52O4/c1-22(2)13-12-19-33(11)27(16-14-23(3)4)21-34(20-18-25(7)8)30(37)28(17-15-24(5)6)31(38)35(33,32(34)39)29(36)26(9)10/h13-15,18,26-28H,12,16-17,19-21H2,1-11H3/t27-,28?,33+,34+,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 596 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM86036
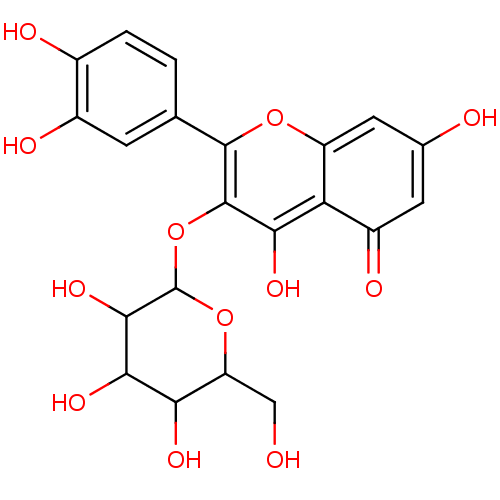
(CAS_5318390 | CAS_97331 | Hyperoside | Miquelianin...)Show SMILES OCC1OC(Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)C(O)C(O)C1O Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 717 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(BOVINE) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 877 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM86036

(CAS_5318390 | CAS_97331 | Hyperoside | Miquelianin...)Show SMILES OCC1OC(Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)C(O)C(O)C1O Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1B
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Rattus norvegicus (Rat)) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM50242259

(CHEMBL516641 | HYPERFORIN)Show SMILES [#6]-[#6](-[#6])-[#6](=O)[C@]12[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-[#6@H](-[#6]\[#6]=[#6](/[#6])-[#6])[C@@]1([#6])[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6]2=O |r,THB:38:37:6.8.14:22.23.29| Show InChI InChI=1S/C35H52O4/c1-22(2)13-12-19-33(11)27(16-14-23(3)4)21-34(20-18-25(7)8)30(37)28(17-15-24(5)6)31(38)35(33,32(34)39)29(36)26(9)10/h13-15,18,26-28H,12,16-17,19-21H2,1-11H3/t27-,28?,33+,34+,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM84979
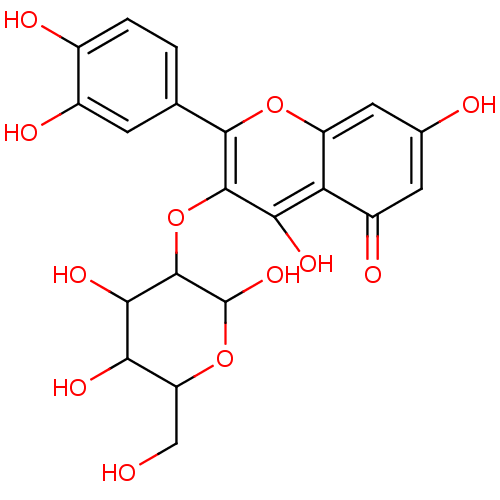
(Isoquercitrin)Show SMILES OCC1OC(O)C(Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)C(O)C1O Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)20(21(30)32-13)33-19-16(28)14-11(26)4-8(23)5-12(14)31-18(19)7-1-2-9(24)10(25)3-7/h1-5,13,15,17,20-25,27-30H,6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Homo sapiens (Human)) | BDBM84978

(Quercitrin | cid_5280459)Show SMILES C[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-24,26-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM84978

(Quercitrin | cid_5280459)Show SMILES C[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-24,26-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50242259

(CHEMBL516641 | HYPERFORIN)Show SMILES [#6]-[#6](-[#6])-[#6](=O)[C@]12[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-[#6@H](-[#6]\[#6]=[#6](/[#6])-[#6])[C@@]1([#6])[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6]2=O |r,THB:38:37:6.8.14:22.23.29| Show InChI InChI=1S/C35H52O4/c1-22(2)13-12-19-33(11)27(16-14-23(3)4)21-34(20-18-25(7)8)30(37)28(17-15-24(5)6)31(38)35(33,32(34)39)29(36)26(9)10/h13-15,18,26-28H,12,16-17,19-21H2,1-11H3/t27-,28?,33+,34+,35+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM84978

(Quercitrin | cid_5280459)Show SMILES C[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-24,26-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(Homo sapiens (Human)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Rattus norvegicus) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50333880

(CHEMBL1614664 | Hypericin | Pseudohypericin)Show SMILES Cc1cc(O)c2c3c1c1c(CO)cc(O)c4c1c1c5c(c(O)cc(O)c5c4=O)c4c(O)cc(O)c(c4c31)c2=O Show InChI InChI=1S/C30H16O9/c1-7-2-9(32)19-23-15(7)16-8(6-31)3-10(33)20-24(16)28-26-18(12(35)5-14(37)22(26)30(20)39)17-11(34)4-13(36)21(29(19)38)25(17)27(23)28/h2-5,31-37H,6H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50242259

(CHEMBL516641 | HYPERFORIN)Show SMILES [#6]-[#6](-[#6])-[#6](=O)[C@]12[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-[#6@H](-[#6]\[#6]=[#6](/[#6])-[#6])[C@@]1([#6])[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6]2=O |r,THB:38:37:6.8.14:22.23.29| Show InChI InChI=1S/C35H52O4/c1-22(2)13-12-19-33(11)27(16-14-23(3)4)21-34(20-18-25(7)8)30(37)28(17-15-24(5)6)31(38)35(33,32(34)39)29(36)26(9)10/h13-15,18,26-28H,12,16-17,19-21H2,1-11H3/t27-,28?,33+,34+,35+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50242259

(CHEMBL516641 | HYPERFORIN)Show SMILES [#6]-[#6](-[#6])-[#6](=O)[C@]12[#6](=O)-[#6](-[#6]\[#6]=[#6](/[#6])-[#6])-[#6](=O)[C@@]([#6]\[#6]=[#6](\[#6])-[#6])([#6]-[#6@H](-[#6]\[#6]=[#6](/[#6])-[#6])[C@@]1([#6])[#6]-[#6]\[#6]=[#6](/[#6])-[#6])[#6]2=O |r,THB:38:37:6.8.14:22.23.29| Show InChI InChI=1S/C35H52O4/c1-22(2)13-12-19-33(11)27(16-14-23(3)4)21-34(20-18-25(7)8)30(37)28(17-15-24(5)6)31(38)35(33,32(34)39)29(36)26(9)10/h13-15,18,26-28H,12,16-17,19-21H2,1-11H3/t27-,28?,33+,34+,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Rattus norvegicus (Rat)) | BDBM50217942

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4-oxo-4H-chr...)Show SMILES C[C@@H]1O[C@@H](OC[C@H]2O[C@@H](Oc3c(O)c4c(cc(O)cc4=O)oc3-c3ccc(O)c(O)c3)[C@H](O)[C@@H](O)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C27H30O16/c1-8-17(32)20(35)22(37)26(40-8)39-7-15-18(33)21(36)23(38)27(42-15)43-25-19(34)16-13(31)5-10(28)6-14(16)41-24(25)9-2-3-11(29)12(30)4-9/h2-6,8,15,17-18,20-23,26-30,32-38H,7H2,1H3/t8-,15+,17-,18+,20+,21-,22+,23+,26+,27-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(RAT) | BDBM86036

(CAS_5318390 | CAS_97331 | Hyperoside | Miquelianin...)Show SMILES OCC1OC(Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)C(O)C(O)C1O Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2 | Reactome pathway
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM86036

(CAS_5318390 | CAS_97331 | Hyperoside | Miquelianin...)Show SMILES OCC1OC(Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)C(O)C(O)C1O Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(BOVINE) | BDBM84978

(Quercitrin | cid_5280459)Show SMILES C[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-24,26-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50129952

(2-(3-(5,7-dihydroxy-2-(4-hydroxyphenyl)-4-oxo-4H-c...)Show SMILES Oc1ccc(cc1)-c1cc(=O)c2c(O)cc(O)c(-c3cc(ccc3O)-c3cc(O)c4c(cc(O)cc4=O)o3)c2o1 |(13.25,-26.01,;14.59,-25.24,;14.59,-23.7,;15.92,-22.93,;17.26,-23.7,;17.26,-25.24,;15.93,-26.01,;18.58,-22.93,;19.91,-23.69,;21.23,-22.92,;22.57,-23.69,;21.22,-21.4,;22.56,-20.64,;23.88,-21.41,;22.55,-19.1,;21.22,-18.33,;21.22,-16.79,;19.9,-19.11,;18.57,-18.34,;17.23,-19.1,;15.9,-18.33,;15.9,-16.79,;17.23,-16.02,;18.57,-16.79,;19.91,-16.02,;14.57,-19.09,;14.56,-20.65,;13.21,-21.42,;13.21,-22.96,;11.87,-20.64,;11.88,-19.08,;10.54,-18.32,;9.21,-19.09,;7.87,-18.32,;9.21,-20.64,;10.54,-21.41,;10.54,-22.95,;13.22,-18.3,;19.9,-20.64,;18.58,-21.4,)| Show InChI InChI=1S/C30H18O10/c31-15-4-1-13(2-5-15)24-12-23(38)29-21(36)10-20(35)27(30(29)40-24)17-7-14(3-6-18(17)33)25-11-22(37)28-19(34)8-16(32)9-26(28)39-25/h1-12,31-33,35-37H | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM86036

(CAS_5318390 | CAS_97331 | Hyperoside | Miquelianin...)Show SMILES OCC1OC(Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)C(O)C(O)C1O Show InChI InChI=1S/C21H20O12/c22-6-13-15(27)17(29)18(30)21(32-13)33-20-16(28)14-11(26)4-8(23)5-12(14)31-19(20)7-1-2-9(24)10(25)3-7/h1-5,13,15,17-18,21-25,27-30H,6H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM84978

(Quercitrin | cid_5280459)Show SMILES C[C@@H]1O[C@@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)c(O)c2)[C@H](O)[C@H](O)[C@H]1O Show InChI InChI=1S/C21H20O11/c1-7-15(26)17(28)18(29)21(30-7)32-20-16(27)14-12(25)5-9(22)6-13(14)31-19(20)8-2-3-10(23)11(24)4-8/h2-7,15,17-18,21-24,26-29H,1H3/t7-,15-,17+,18+,21-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Westfälische Wilhelms-Universitä Muenster,
Curated by PDSP Ki Database
| |
Psychopharmacology (Berl) 162: 193-202 (2002)
Article DOI: 10.1007/s00213-002-1073-7
BindingDB Entry DOI: 10.7270/Q25D8QDD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data