

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50117263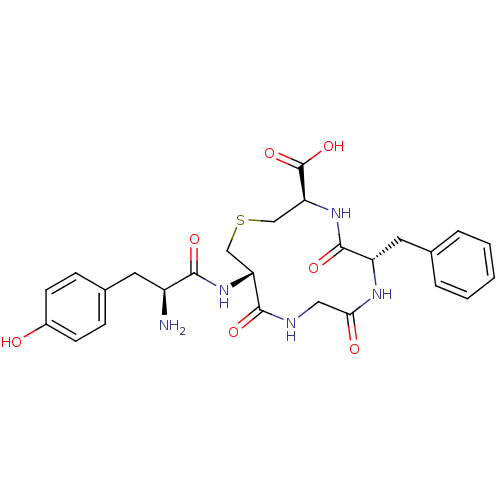 ((3R,6S,12S)-12-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor delta 1 expressed in CHO cell membrane;Range is in between (0.62-0.65) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50117262 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor delta 1 expressed in CHO cell membrane;Range is between (0.66-0.95) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50117264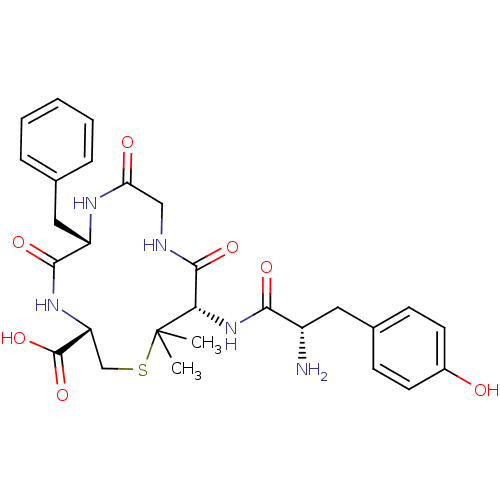 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor delta 1 expressed in CHO cell membrane;Range is between (0.35-2.5) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50117261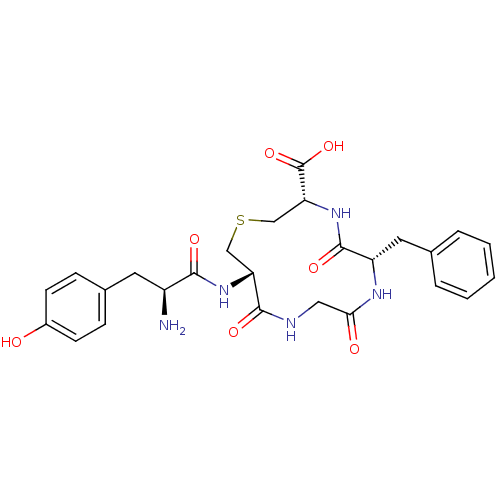 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor delta 1 expressed in CHO cell membrane;Range is between (1.7-2.3) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50117261 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor mu 1 expressed in CHO cell membrane;Range is in between (1.8-2.2) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor delta 1 expressed in CHO cell membrane;Range is between (1-3.9) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50117263 ((3R,6S,12S)-12-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor mu 1 expressed in CHO cell membrane;Range is in between (1.3-3.7) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor mu 1 expressed in CHO cell membrane;Range is in between (6.8-43) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50117262 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor mu 1 expressed in CHO cell membrane;Range is in between (110-150) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor delta 1 expressed in CHO cell membrane;Range is between (63-360) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor kappa 1 expressed in CHO cell membrane;Range is in between (1400-1900) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50117264 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor mu 1 expressed in CHO cell membrane;Range is in between (580-680) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50117262 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor kappa 1 expressed in CHO cell membrane;Range is in between (1400-1900) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50117261 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor kappa 1 expressed in CHO cell membrane;Range is in between (1400-1900) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50117263 ((3R,6S,12S)-12-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-diprenorphine to cloned human Opioid receptor kappa 1 expressed in CHO cell membrane; range is in between (4700-8500) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor kappa 1 expressed in CHO cell membrane | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50117264 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor kappa 1 expressed in CHO cell membrane;Range is in between (1400-1900) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of binding of [3H]diprenorphine to cloned human Opioid receptor mu 1 expressed in CHO cell membrane | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50117262 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of mouse vas deference (MVD, delta receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50117263 ((3R,6S,12S)-12-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of mouse vas deferens (MVD, delta receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50117261 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of guinea pig ileum (GPI, mu receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50117263 ((3R,6S,12S)-12-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of guinea pig ileum (GPI, mu receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50117261 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of mouse vas deferens (MVD, delta receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50117264 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of mouse vas deference (MVD, delta receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of mouse vas deference (MVD, delta receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of guinea pig ileum (GPI, mu receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50117262 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of guinea pig ileum (GPI, mu receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000092 ((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 644 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of mouse vas deference (MVD, delta receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50117264 (12-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-6...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of guinea pig ileum (GPI, mu receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Ability to inhibit electrically evoked contractions of isolated muscle preparations of guinea pig ileum (GPI, mu receptor) | J Med Chem 45: 3746-54 (2002) BindingDB Entry DOI: 10.7270/Q2RN376Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||