

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50409654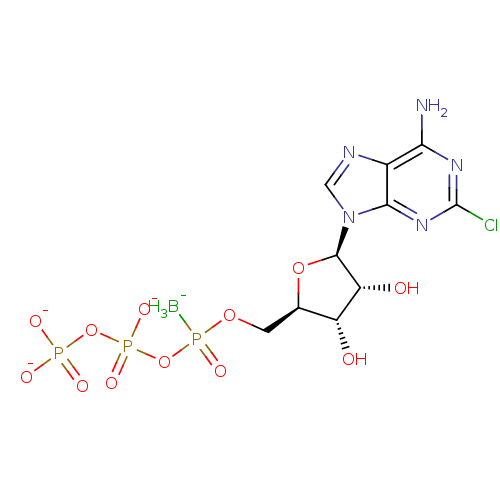 (CHEMBL2021421) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50267999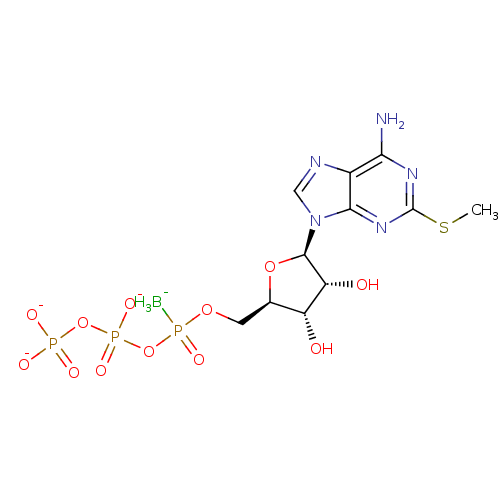 (({[({[(2R,3S,4R,5R)-5-[6-amino-2-(methylsulfanyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50422408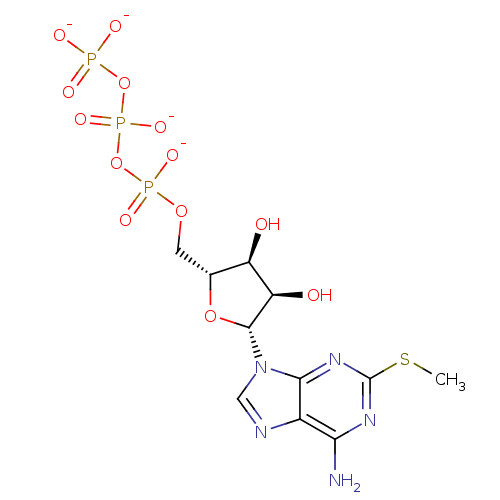 (CHEMBL2364736) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50422407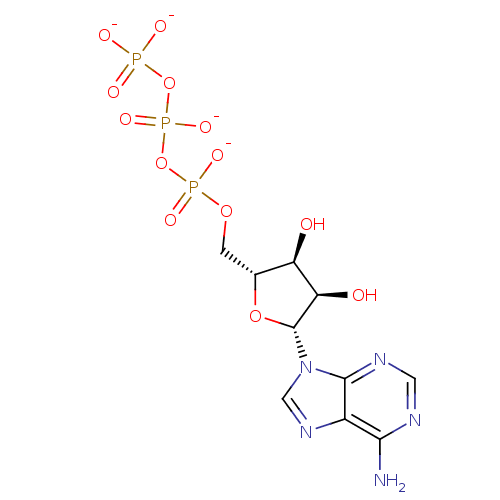 (CHEMBL2364735) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50370180 (CHEMBL607612) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 1 (Rattus norvegicus) | BDBM50422406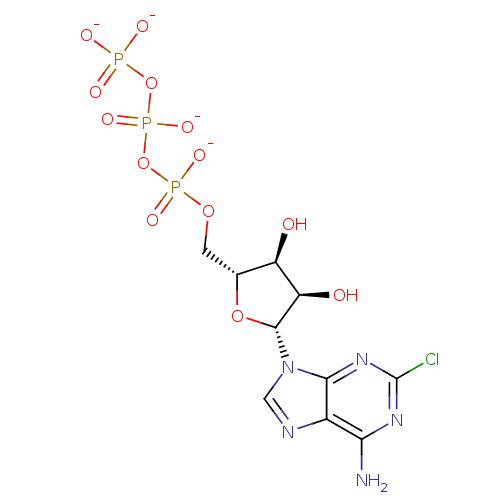 (2-Chloroadenosine Triphosphate Tetrasodium | CHEMB...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a |
Bar-Ilan University Curated by ChEMBL | Assay Description Agonists activity was evaluated by release of [Ca2+] release of HEK 293 cells stably transfected with rat-brain P2Y purinoceptor 1 (P2Y1-R) | J Med Chem 45: 5384-96 (2002) BindingDB Entry DOI: 10.7270/Q2M909DD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||