 Found 30 hits of Enzyme Inhibition Constant Data
Found 30 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121950
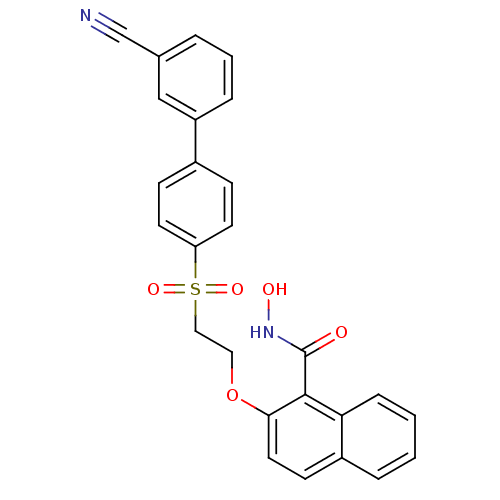
(2-[2-(3'-Cyano-biphenyl-4-sulfonyl)-ethoxy]-naphth...)Show SMILES ONC(=O)c1c(OCCS(=O)(=O)c2ccc(cc2)-c2cccc(c2)C#N)ccc2ccccc12 Show InChI InChI=1S/C26H20N2O5S/c27-17-18-4-3-6-21(16-18)19-8-11-22(12-9-19)34(31,32)15-14-33-24-13-10-20-5-1-2-7-23(20)25(24)26(29)28-30/h1-13,16,30H,14-15H2,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121945

(2-[3-(4'-Methoxy-biphenyl-4-sulfonyl)-propyl]-naph...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CCCc1ccc2ccccc2c1C(=O)NO Show InChI InChI=1S/C27H25NO5S/c1-33-23-14-10-19(11-15-23)20-12-16-24(17-13-20)34(31,32)18-4-6-22-9-8-21-5-2-3-7-25(21)26(22)27(29)28-30/h2-3,5,7-17,30H,4,6,18H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121944
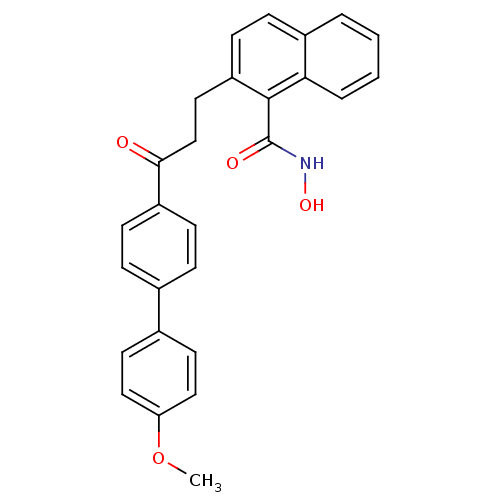
(2-[3-(4'-Methoxy-biphenyl-4-yl)-3-oxo-propyl]-naph...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)CCc1ccc2ccccc2c1C(=O)NO Show InChI InChI=1S/C27H23NO4/c1-32-23-15-12-19(13-16-23)18-6-9-21(10-7-18)25(29)17-14-22-11-8-20-4-2-3-5-24(20)26(22)27(30)28-31/h2-13,15-16,31H,14,17H2,1H3,(H,28,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121946
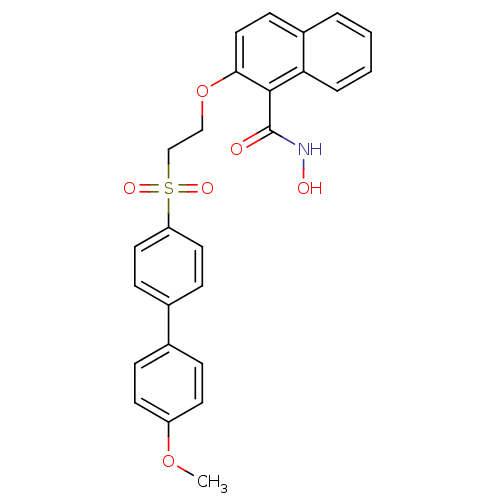
(2-[2-(4'-Methoxy-biphenyl-4-sulfonyl)-ethoxy]-naph...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CCOc1ccc2ccccc2c1C(=O)NO Show InChI InChI=1S/C26H23NO6S/c1-32-21-11-6-18(7-12-21)19-8-13-22(14-9-19)34(30,31)17-16-33-24-15-10-20-4-2-3-5-23(20)25(24)26(28)27-29/h2-15,29H,16-17H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121959
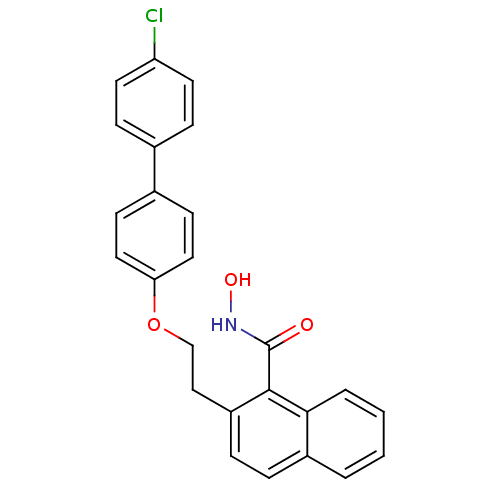
(2-[2-(4'-Chloro-biphenyl-4-yloxy)-ethyl]-naphthale...)Show SMILES ONC(=O)c1c(CCOc2ccc(cc2)-c2ccc(Cl)cc2)ccc2ccccc12 Show InChI InChI=1S/C25H20ClNO3/c26-21-11-7-17(8-12-21)18-9-13-22(14-10-18)30-16-15-20-6-5-19-3-1-2-4-23(19)24(20)25(28)27-29/h1-14,29H,15-16H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121942
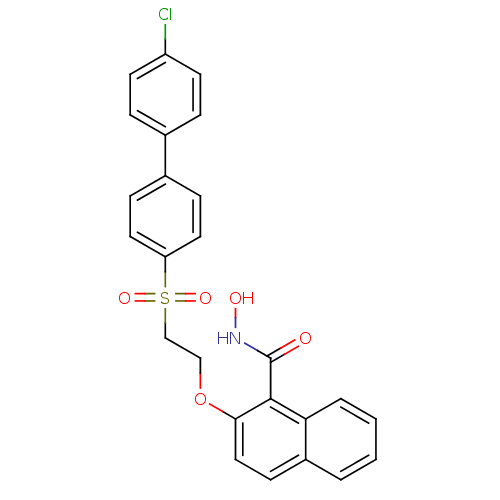
(2-[2-(4'-Chloro-biphenyl-4-sulfonyl)-ethoxy]-napht...)Show SMILES ONC(=O)c1c(OCCS(=O)(=O)c2ccc(cc2)-c2ccc(Cl)cc2)ccc2ccccc12 Show InChI InChI=1S/C25H20ClNO5S/c26-20-10-5-17(6-11-20)18-7-12-21(13-8-18)33(30,31)16-15-32-23-14-9-19-3-1-2-4-22(19)24(23)25(28)27-29/h1-14,29H,15-16H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 670 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121951
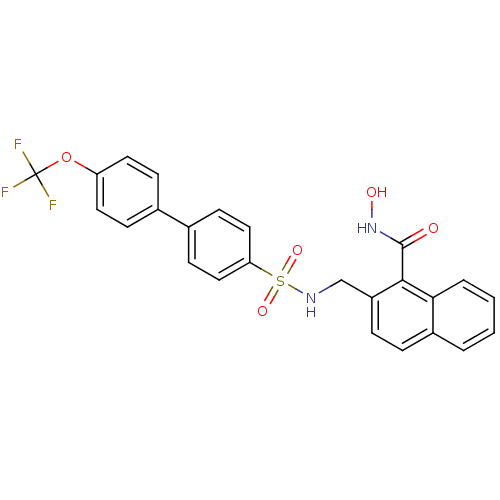
(2-[(4'-Trifluoromethoxy-biphenyl-4-sulfonylamino)-...)Show SMILES ONC(=O)c1c(CNS(=O)(=O)c2ccc(cc2)-c2ccc(OC(F)(F)F)cc2)ccc2ccccc12 Show InChI InChI=1S/C25H19F3N2O5S/c26-25(27,28)35-20-11-7-16(8-12-20)17-9-13-21(14-10-17)36(33,34)29-15-19-6-5-18-3-1-2-4-22(18)23(19)24(31)30-32/h1-14,29,32H,15H2,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121961

(2-[4-(4'-Methoxy-biphenyl-4-yl)-4-oxo-butyl]-napht...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)C(=O)CCCc1ccc2ccccc2c1C(=O)NO Show InChI InChI=1S/C28H25NO4/c1-33-24-17-15-20(16-18-24)19-9-12-22(13-10-19)26(30)8-4-6-23-14-11-21-5-2-3-7-25(21)27(23)28(31)29-32/h2-3,5,7,9-18,32H,4,6,8H2,1H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121954

(2-[2-(4'-Chloro-biphenyl-4-sulfonyl)-ethyl]-naphth...)Show SMILES ONC(=O)c1c(CCS(=O)(=O)c2ccc(cc2)-c2ccc(Cl)cc2)ccc2ccccc12 Show InChI InChI=1S/C25H20ClNO4S/c26-21-11-7-17(8-12-21)18-9-13-22(14-10-18)32(30,31)16-15-20-6-5-19-3-1-2-4-23(19)24(20)25(28)27-29/h1-14,29H,15-16H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121947

(2-(4'-Cyano-biphenyl-4-yloxymethyl)-naphthalene-1-...)Show SMILES ONC(=O)c1c(COc2ccc(cc2)-c2ccc(cc2)C#N)ccc2ccccc12 Show InChI InChI=1S/C25H18N2O3/c26-15-17-5-7-18(8-6-17)19-11-13-22(14-12-19)30-16-21-10-9-20-3-1-2-4-23(20)24(21)25(28)27-29/h1-14,29H,16H2,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121948
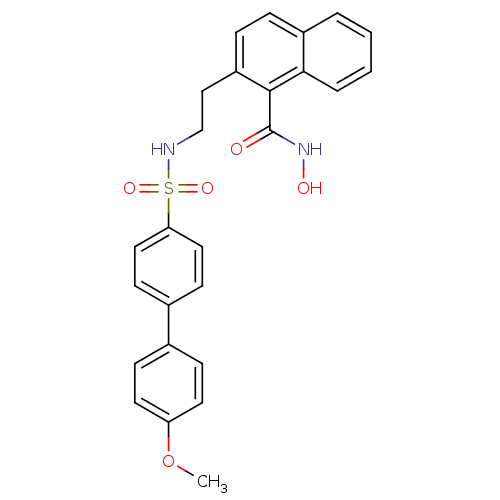
(2-[2-(4'-Methoxy-biphenyl-4-sulfonylamino)-ethyl]-...)Show SMILES COc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NCCc1ccc2ccccc2c1C(=O)NO Show InChI InChI=1S/C26H24N2O5S/c1-33-22-12-8-18(9-13-22)19-10-14-23(15-11-19)34(31,32)27-17-16-21-7-6-20-4-2-3-5-24(20)25(21)26(29)28-30/h2-15,27,30H,16-17H2,1H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121962

(2-[3-(4'-Cyano-biphenyl-4-yloxy)-propoxy]-naphthal...)Show SMILES ONC(=O)c1c(OCCCOc2ccc(cc2)-c2ccc(cc2)C#N)ccc2ccccc12 Show InChI InChI=1S/C27H22N2O4/c28-18-19-6-8-20(9-7-19)21-10-13-23(14-11-21)32-16-3-17-33-25-15-12-22-4-1-2-5-24(22)26(25)27(30)29-31/h1-2,4-15,31H,3,16-17H2,(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015152
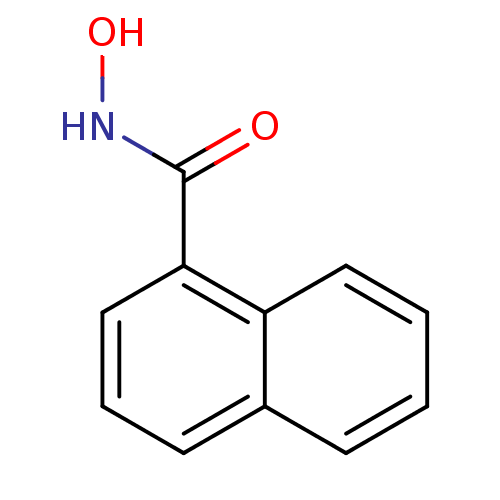
(CHEMBL115468 | N-hydroxy-1-naphthamide | Naphthale...)Show InChI InChI=1S/C11H9NO2/c13-11(12-14)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,14H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Stromelysin (MMP-3) |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121953
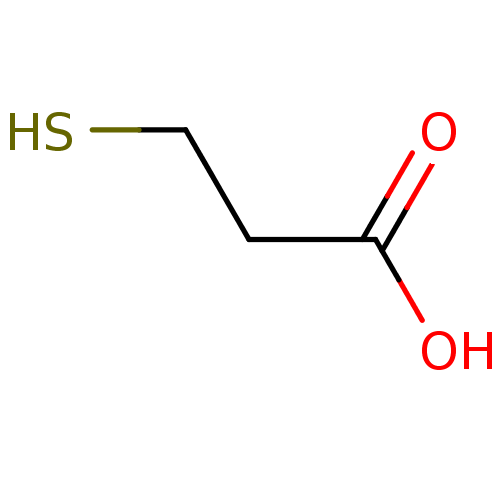
(2-mercaptoethanecarboxylic acid | 3-mercaptopropan...)Show InChI InChI=1S/C3H6O2S/c4-3(5)1-2-6/h6H,1-2H2,(H,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 3.00E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121957
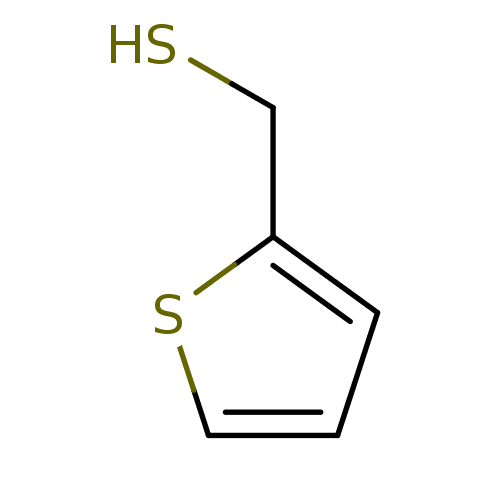
(CHEMBL152603 | Thiophen-2-yl-methanethiol)Show InChI InChI=1S/C5H6S2/c6-4-5-2-1-3-7-5/h1-3,6H,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121958

(4,4'-Biphenyldiol | 4,4'-Dihydroxybiphenyl | 4,4'-...)Show InChI InChI=1S/C12H10O2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8,13-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.60E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015152

(CHEMBL115468 | N-hydroxy-1-naphthamide | Naphthale...)Show InChI InChI=1S/C11H9NO2/c13-11(12-14)10-7-3-5-8-4-1-2-6-9(8)10/h1-7,14H,(H,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121958

(4,4'-Biphenyldiol | 4,4'-Dihydroxybiphenyl | 4,4'-...)Show InChI InChI=1S/C12H10O2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8,13-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 7.00E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50099857
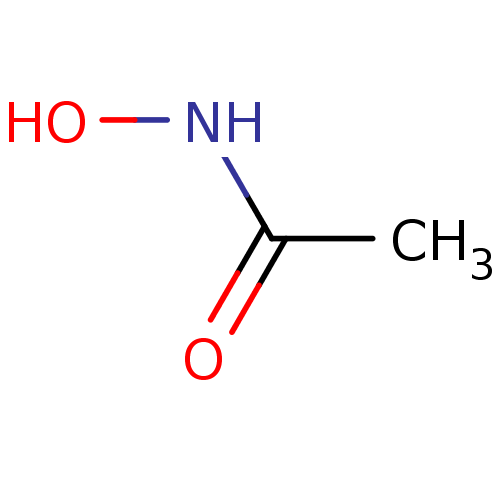
(ACETOHYDROXAMIC ACID (AHA) | AHA | Acethydroxamsae...)Show InChI InChI=1S/C2H5NO2/c1-2(4)3-5/h5H,1H3,(H,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | 1.70E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50056900
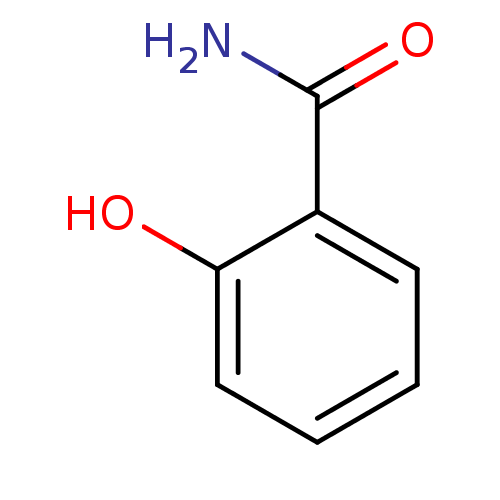
(2-Carbamoylphenol | 2-Carboxamidophenol | 2-Hydrox...)Show InChI InChI=1S/C7H7NO2/c8-7(10)5-3-1-2-4-6(5)9/h1-4,9H,(H2,8,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121952
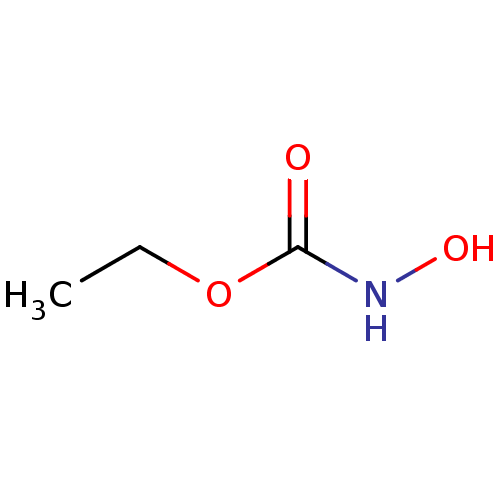
(CHEMBL153081 | Ethyl hydroxycarbamate)Show InChI InChI=1S/C3H7NO3/c1-2-7-3(5)4-6/h6H,2H2,1H3,(H,4,5) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50017811
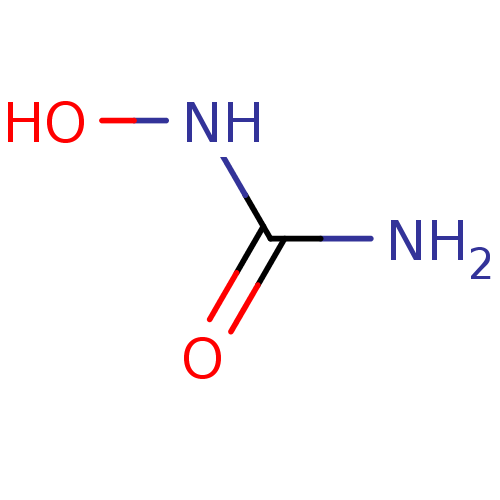
(CHEMBL467 | HU | US10155732, Compound HU | hydroxy...)Show InChI InChI=1S/CH4N2O2/c2-1(4)3-5/h5H,(H3,2,3,4) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM26193
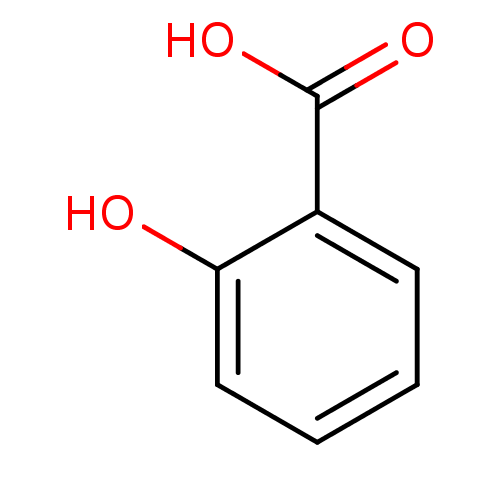
(2-Hydroxybenzoate, I | 2-hydroxybenzoic acid | CHE...)Show InChI InChI=1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121955
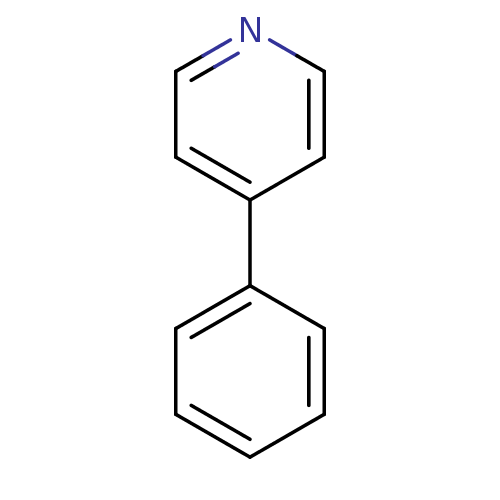
(4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...)Show InChI InChI=1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of acetohydroxamic acid |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121956
(4,4,4-Trifluoro-3-oxo-butyric acid ethyl ester | C...)Show InChI InChI=1S/C6H7F3O3/c1-2-12-5(11)3-4(10)6(7,8)9/h2-3H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50026891

(1-Pyridin-2-yl-ethanone | CHEMBL11945)Show InChI InChI=1S/C7H7NO/c1-6(9)7-4-2-3-5-8-7/h2-5H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121955

(4-Phenyl-pyridine | CHEMBL109074 | US11634391, Com...)Show InChI InChI=1S/C11H9N/c1-2-4-10(5-3-1)11-6-8-12-9-7-11/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 9.00E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in the presence of 1-Napthohydroxamate |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121943
(4'-Hydroxy-biphenyl-4-carbonitrile | CHEMBL114523)Show InChI InChI=1S/C13H9NO/c14-9-10-1-3-11(4-2-10)12-5-7-13(15)8-6-12/h1-8,15H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50121960

(4,4,4-Trifluoro-1-phenyl-butane-1,3-dione | 4,4,4-...)Show InChI InChI=1S/C10H7F3O2/c11-10(12,13)9(15)6-8(14)7-4-2-1-3-5-7/h1-5H,6H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | >2.50E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding to stromelysin (MMP-3) in place of acetohydroxamic acid. |
J Med Chem 45: 5628-39 (2002)
BindingDB Entry DOI: 10.7270/Q20C4V3M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data