

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122970 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122969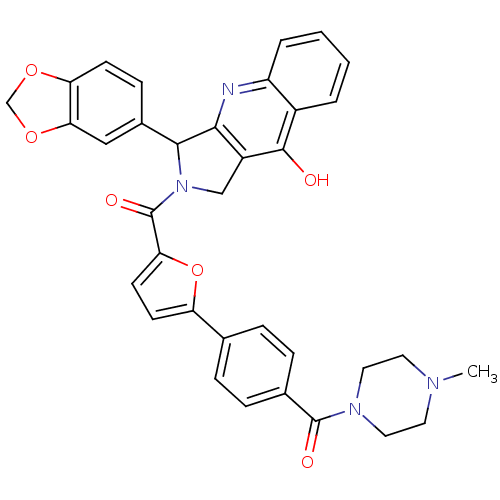 (3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122990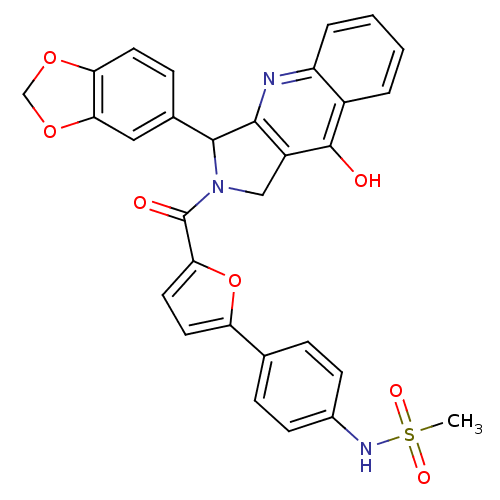 (CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122974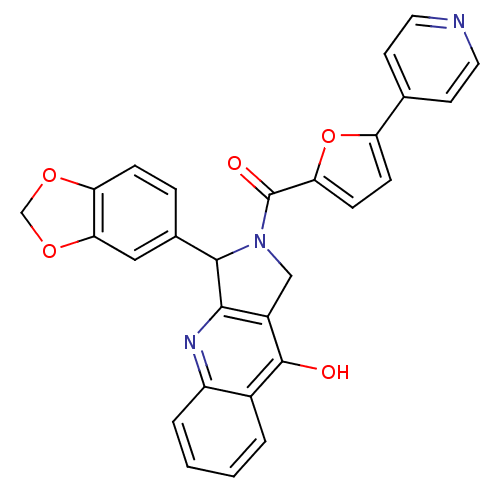 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-4-yl-furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122964 (3-Benzo[1,3]dioxol-5-yl-2-(6-hydroxy-benzofuran-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122966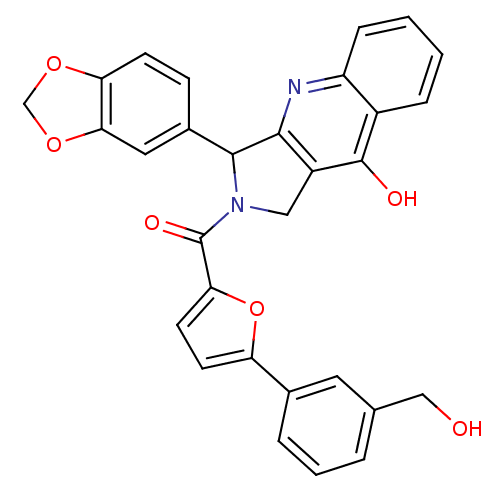 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-hydroxymethyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122971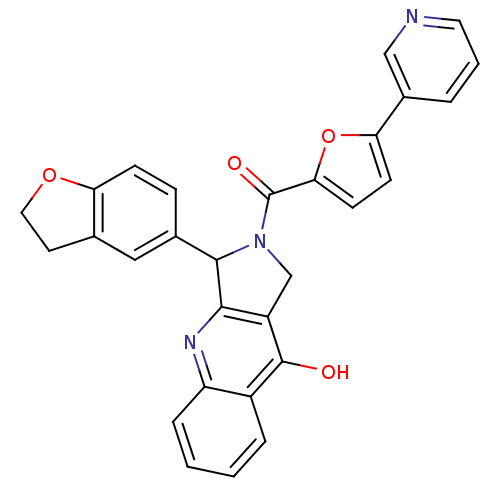 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122973 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxymethyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122983 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-nitro-phenyl)-fura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122980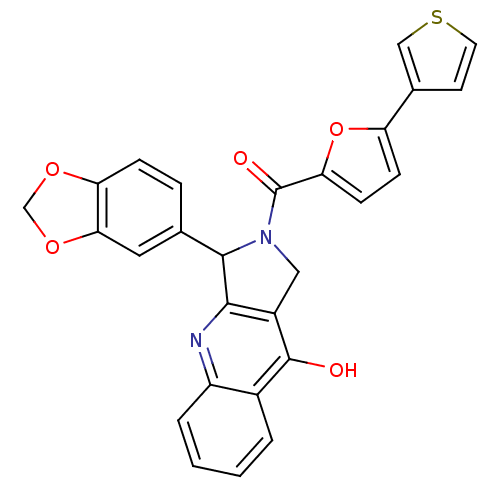 (3-Benzo[1,3]dioxol-5-yl-2-(5-thiophen-3-yl-furan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122981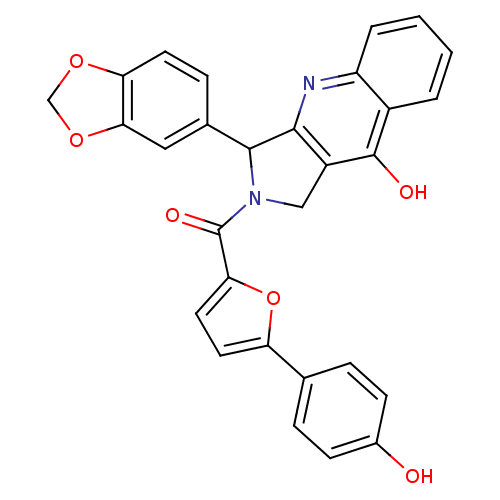 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxy-phenyl)-fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122987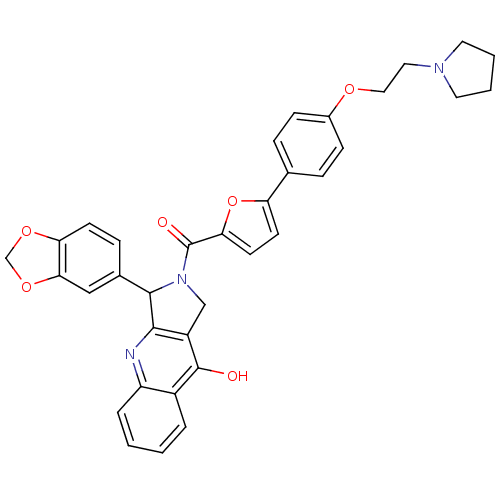 (3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(2-pyrrolidin-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122989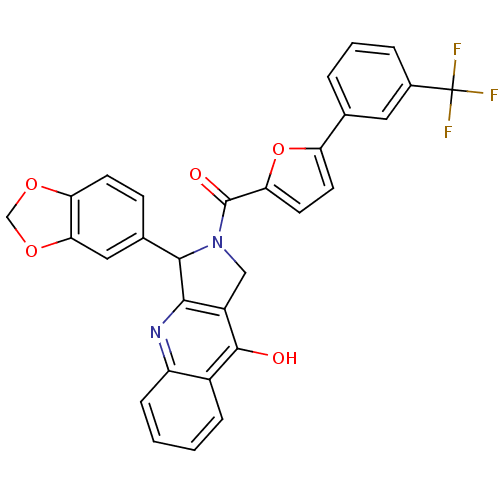 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122967 (4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122976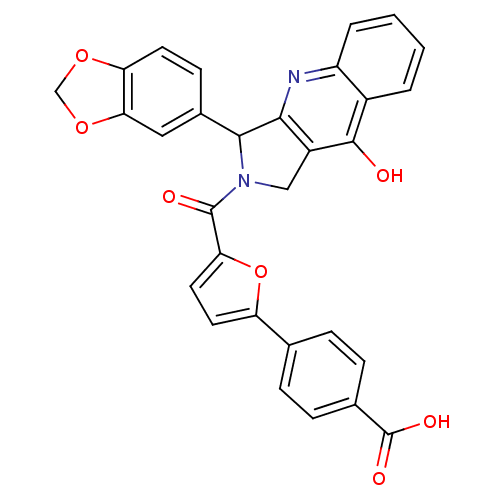 (4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122982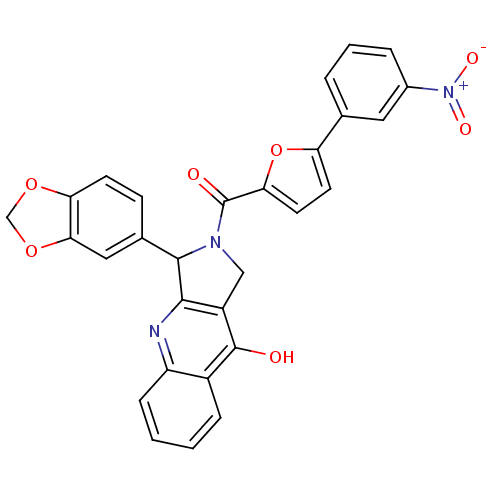 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-nitro-phenyl)-fura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122988 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122985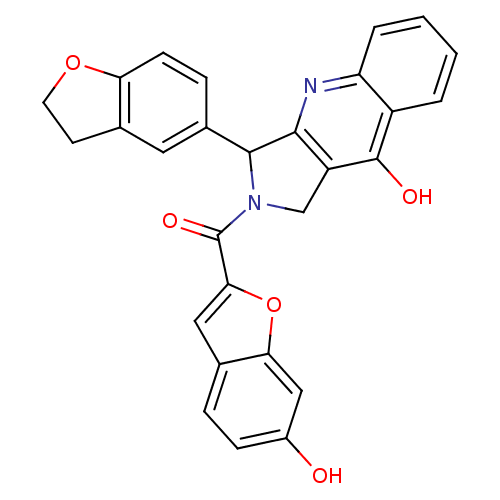 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(6-hydroxy-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122986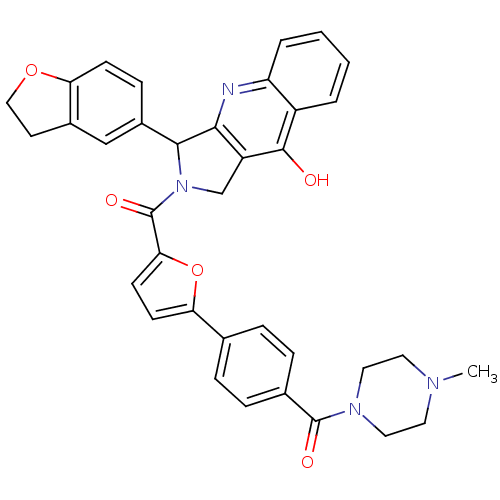 (3-(2,3-Dihydro-benzofuran-5-yl)-2-{5-[4-(4-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122978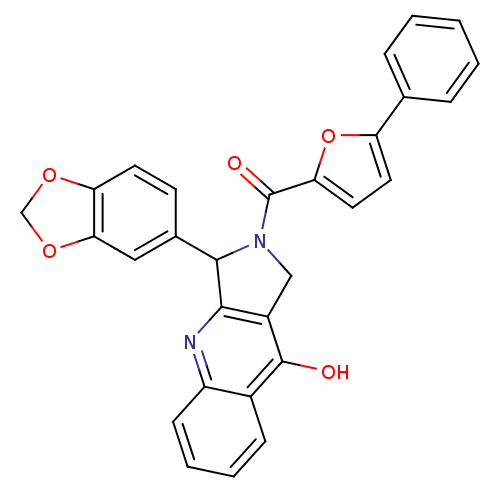 (3-Benzo[1,3]dioxol-5-yl-2-(5-phenyl-furan-2-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122984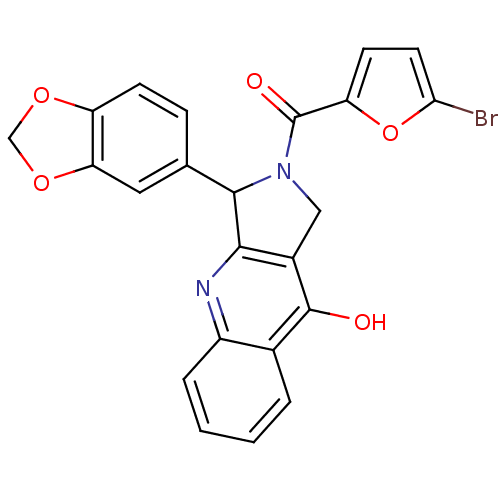 (3-Benzo[1,3]dioxol-5-yl-2-(5-bromo-furan-2-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122979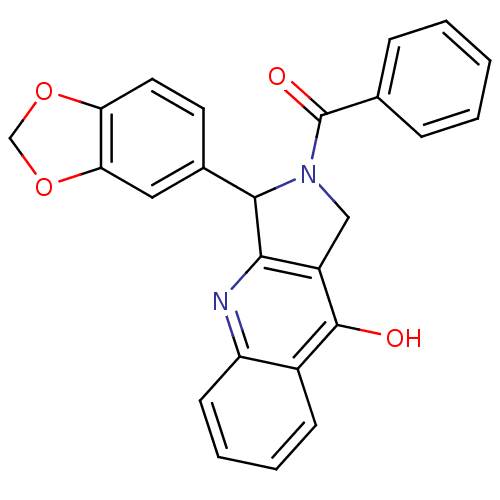 (3-Benzo[1,3]dioxol-5-yl-2-benzoyl-1,2,3,4-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122975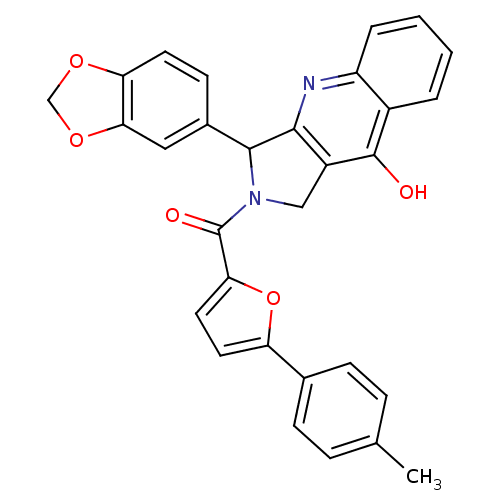 (3-Benzo[1,3]dioxol-5-yl-2-(5-p-tolyl-furan-2-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122968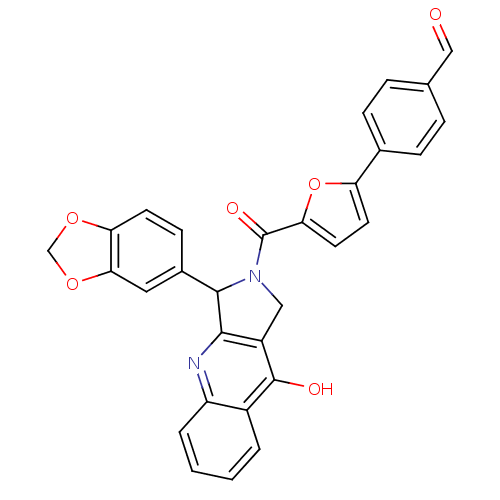 (4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of PDE5 | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122977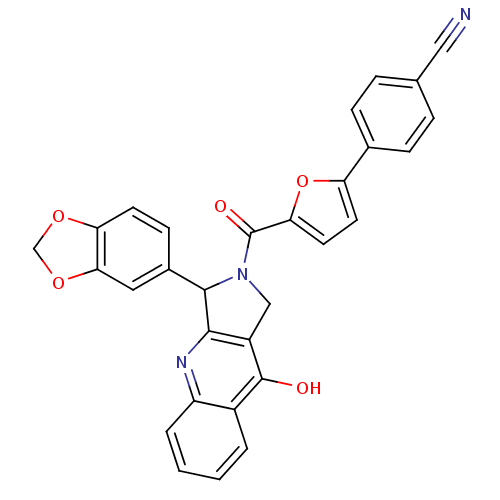 (4-[5-(3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122963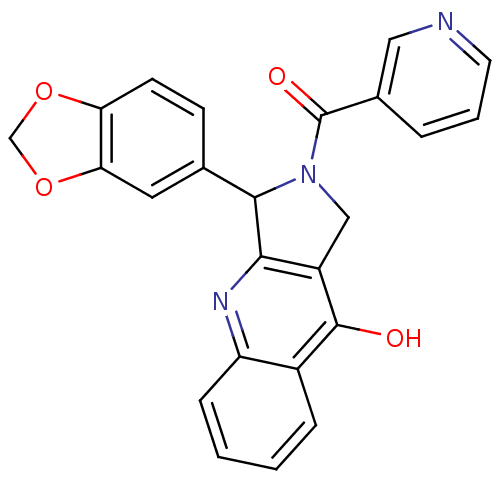 (3-Benzo[1,3]dioxol-5-yl-2-(pyridine-3-carbonyl)-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122965 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-dimethylamino-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122972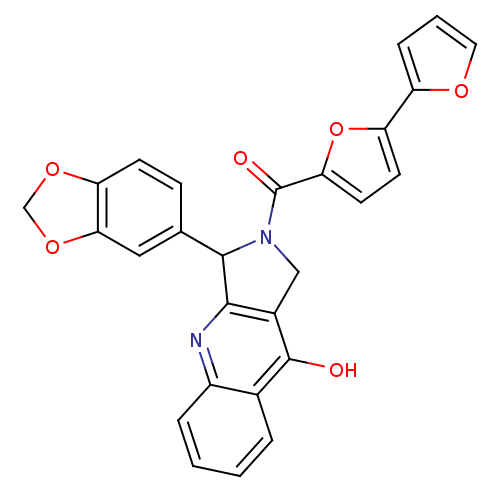 (3-Benzo[1,3]dioxol-5-yl-2-([2,2']bifuranyl-5-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||