

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000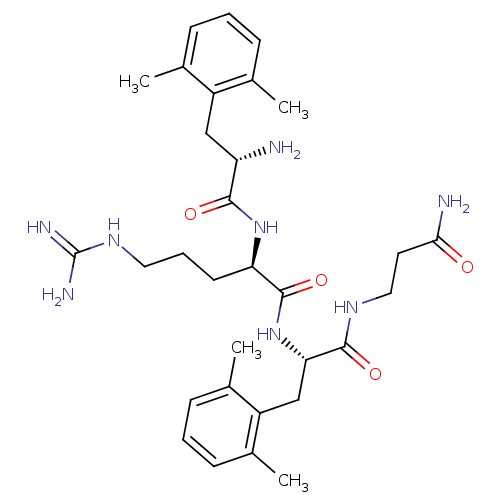 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0216 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126003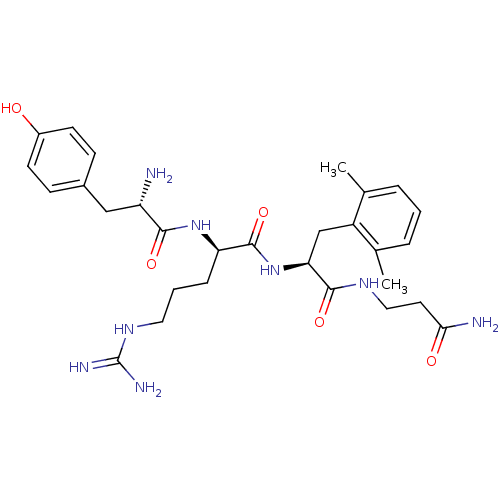 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125997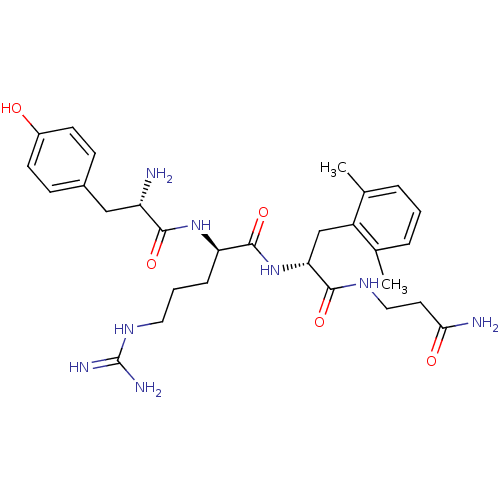 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0618 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0623 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126001 ((R)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126002 ((R)-2-[(R)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-DAMGO from mu opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 482 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126003 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 544 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126000 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126001 ((R)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50126002 ((R)-2-[(R)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50125997 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to displace [3H]-deltorphin II from delta opioid receptor | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50125999 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50126003 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50126000 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50125997 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50126003 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50126000 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50125998 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50125996 ((R)-2-[(S)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 188 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50125997 ((R)-2-[(S)-2-Amino-3-(4-hydroxy-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 305 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50126002 ((R)-2-[(R)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50126001 ((R)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 633 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of guinea pig ileum having mu opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50126002 ((R)-2-[(R)-2-Amino-3-(2,6-dimethyl-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50126001 ((R)-2-((S)-2-Amino-3-phenyl-propionylamino)-5-guan...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Tohoku Pharmaceutical University Curated by ChEMBL | Assay Description Ability of the compound to inhibit electrically induced contractions of mouse vas deferens having delta opioid receptors tested in vitro | Bioorg Med Chem Lett 13: 1269-72 (2003) BindingDB Entry DOI: 10.7270/Q2XS5TQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||