

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13975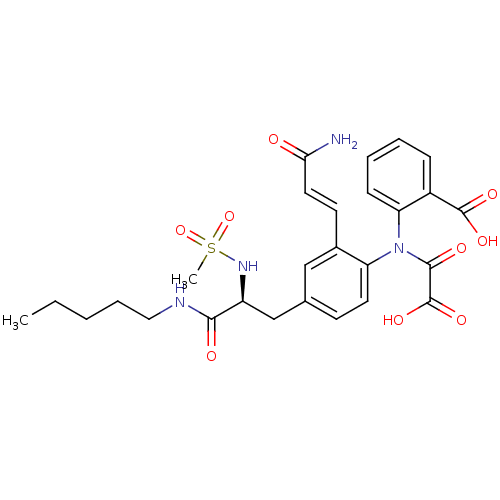 (2-({2-[(1E)-2-carbamoyleth-1-en-1-yl]-4-[(2S)-2-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13975 (2-({2-[(1E)-2-carbamoyleth-1-en-1-yl]-4-[(2S)-2-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13971 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13953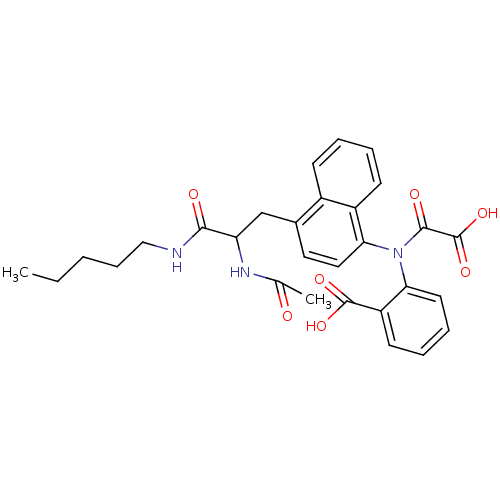 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13953 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13971 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13972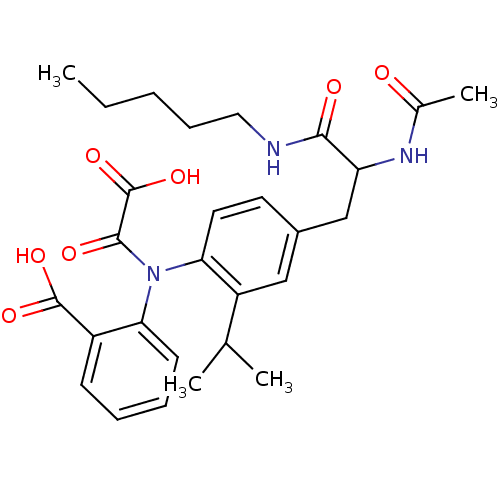 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13972 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13973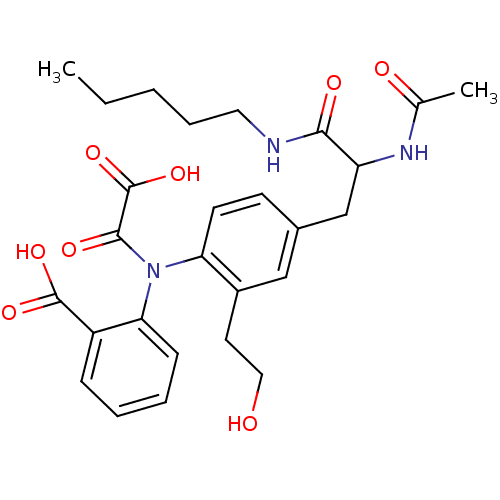 (2-({4-[2-acetamido-2-(pentylcarbamoyl)ethyl]-2-(2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13973 (2-({4-[2-acetamido-2-(pentylcarbamoyl)ethyl]-2-(2-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13974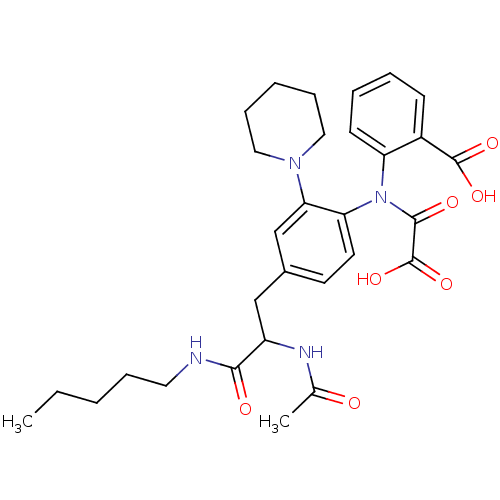 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13969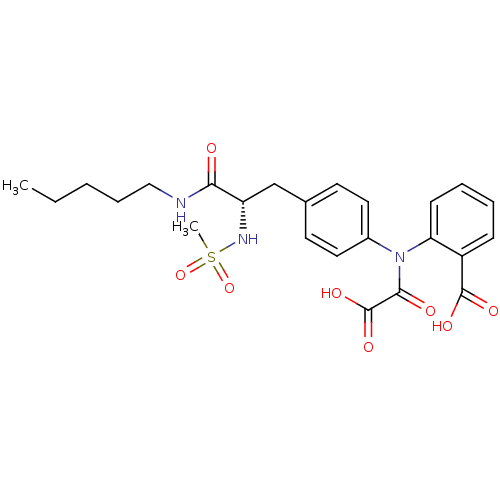 (2-({4-[(2S)-2-methanesulfonamido-2-(pentylcarbamoy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.10E+3 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13968 (2-({4-[(2S)-2-acetamido-2-(pentylcarbamoyl)ethyl]p...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | -28.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13968 (2-({4-[(2S)-2-acetamido-2-(pentylcarbamoyl)ethyl]p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13964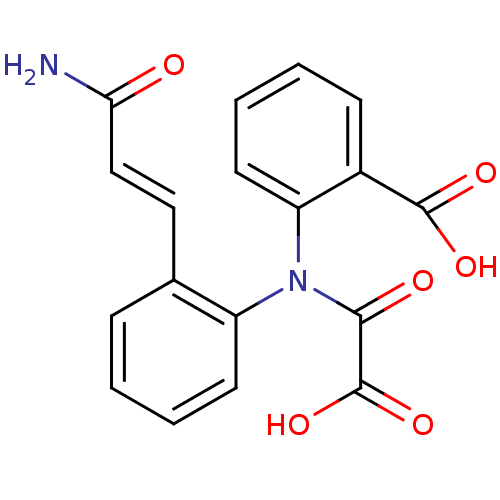 (2-({2-[(1E)-2-carbamoyleth-1-en-1-yl]phenyl}amidof...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.10E+4 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13966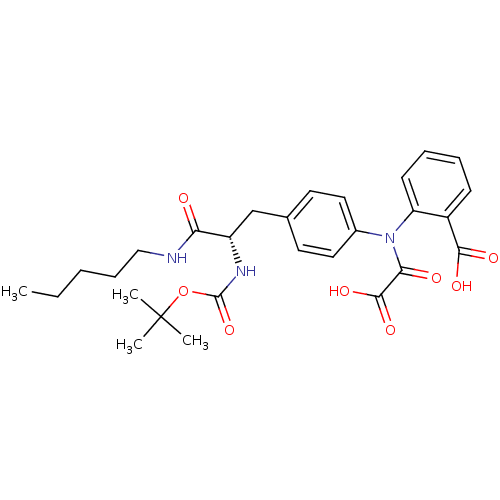 (2-({4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-(pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13965 (2-[Oxalyl(2-piperidin-1-ylphenyl)amino]benzoic Aci...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | -27.4 | n/a | 1.30E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13963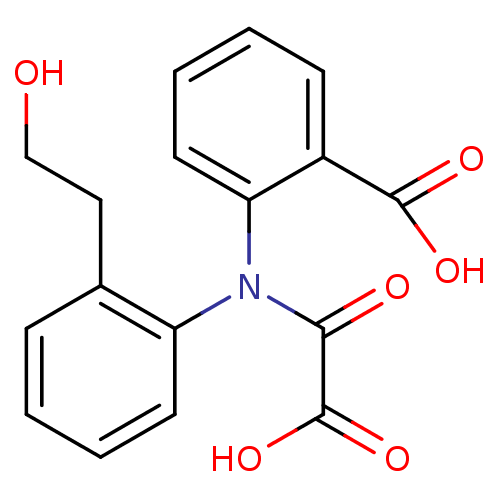 (2-{[2-(2-Hydroxyethyl)phenyl]oxalylamino}benzoic A...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | -26.9 | n/a | 3.50E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13958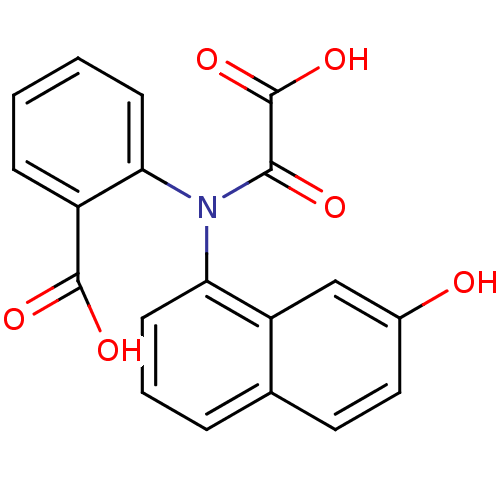 (2-[(7-Hydroxynaphthalen-1-yl)oxalylamino]benzoic A...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 1.70E+4 | -26.9 | n/a | 8.00E+3 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13966 (2-({4-[(2S)-2-{[(tert-butoxy)carbonyl]amino}-2-(pe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.73E+4 | -26.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13952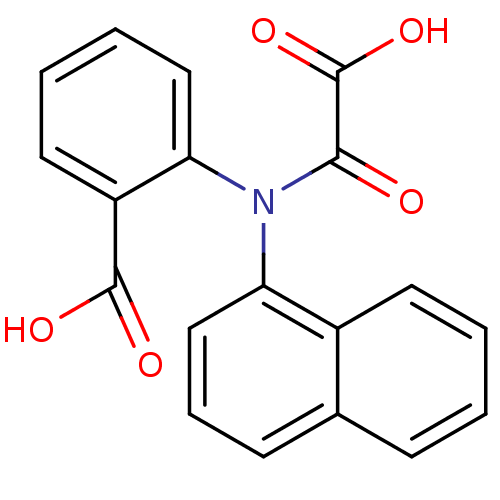 (2-(Naphthalen-1-yloxalylamino)benzoic Acid | 2-(na...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 2.40E+4 | -26.1 | n/a | 2.60E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13962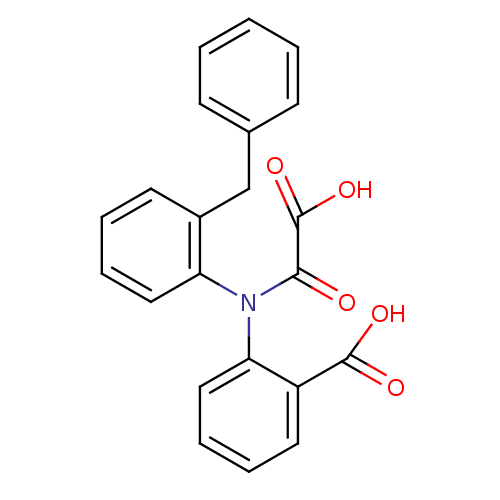 (2-[(2-Benzylphenyl)oxalylamino]benzoic Acid | 2-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.40E+4 | -26.1 | n/a | 3.50E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13961 (2-[(2-Isopropylphenyl)oxalylamino]benzoic Acid | 2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | -25.2 | n/a | 4.90E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13957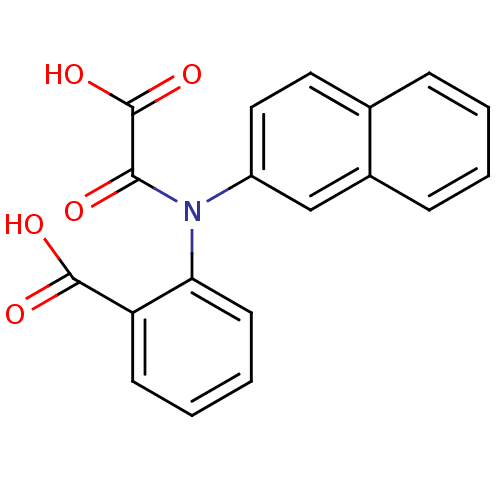 (2-(Naphthalen-2-yloxalylamino)benzoic Acid | 2-(na...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90E+4 | -24.9 | n/a | 1.10E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13960 (2-[(2-Ethylphenyl)oxalylamino]benzoic Acid | 2-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | -23.9 | n/a | 5.00E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13959 (2-(5,6,7,8-tetrahydronaphthalen-1-ylamidoformic ac...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40E+4 | -23.3 | n/a | 7.10E+4 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13951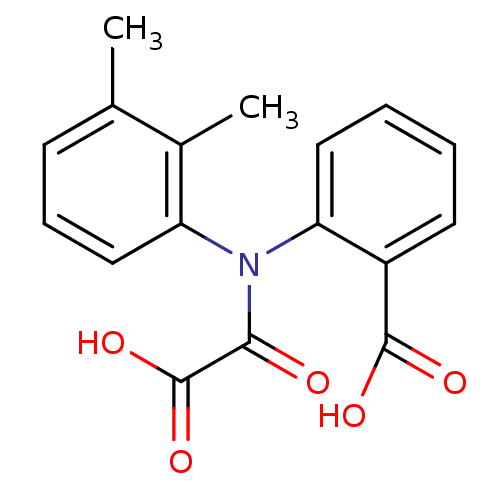 (2-[(2,3-Dimethylphenyl)oxalylamino]benzoic acid | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30E+4 | -22.8 | n/a | 1.24E+5 | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13967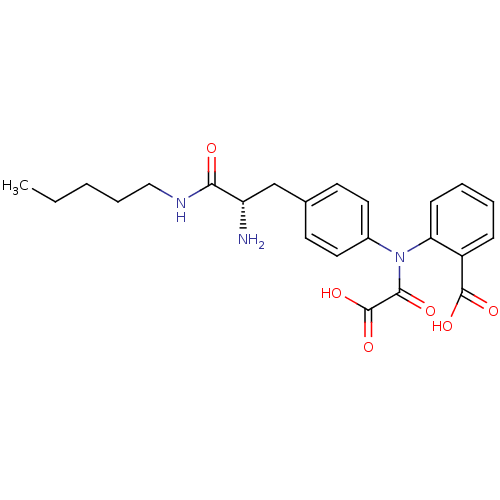 (2-({4-[(2S)-2-amino-2-(pentylcarbamoyl)ethyl]pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13967 (2-({4-[(2S)-2-amino-2-(pentylcarbamoyl)ethyl]pheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | >-22.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | J Med Chem 46: 2093-103 (2003) Article DOI: 10.1021/jm0205696 BindingDB Entry DOI: 10.7270/Q26H4FPH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||