

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15819 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 76 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15817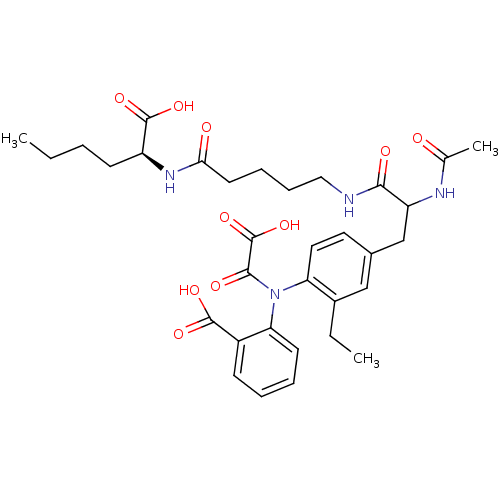 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 120 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15818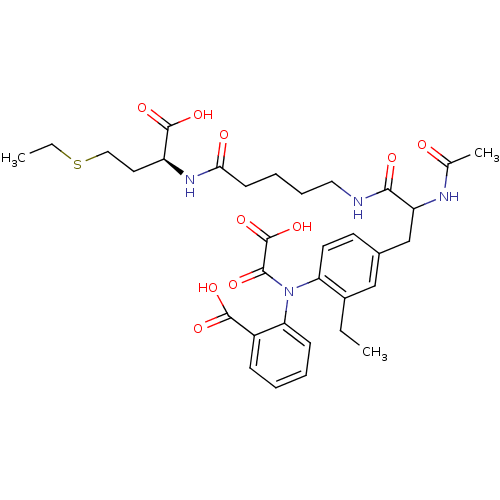 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 130 | -38.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15812 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15812 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15817 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15813 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 250 | -37.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15815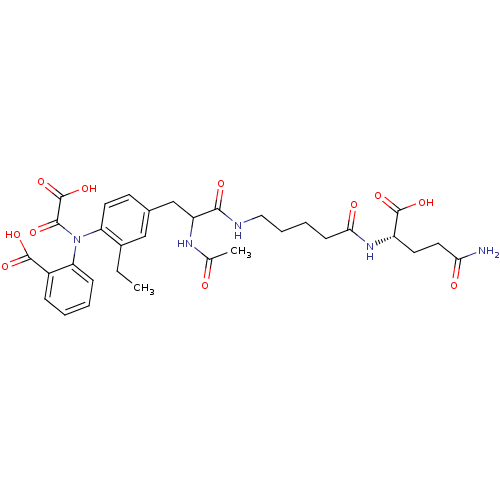 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15813 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(S)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15815 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 330 | -36.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15820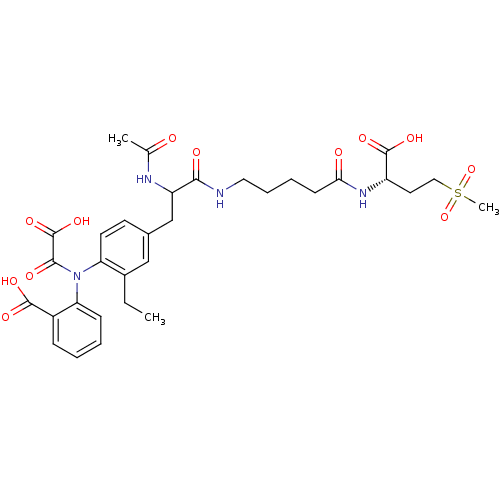 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15819 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15814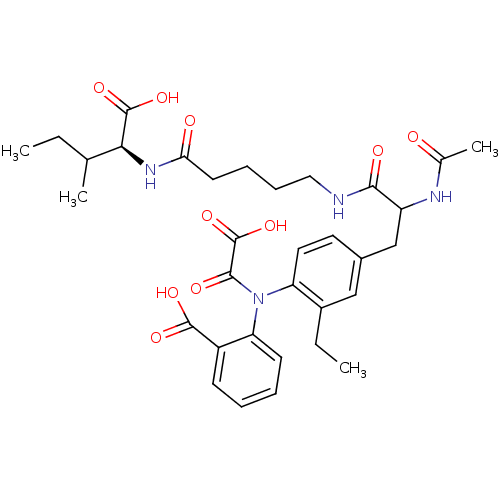 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15814 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 430 | -36.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15806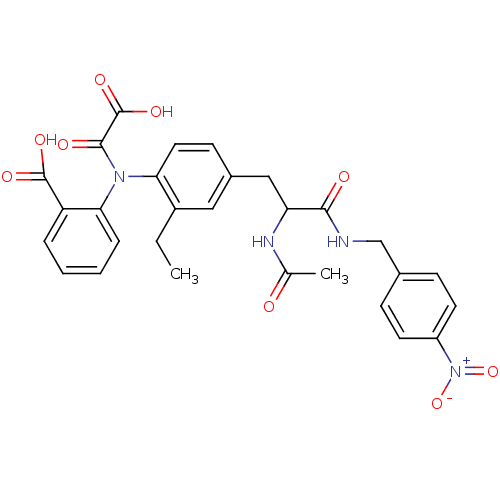 (1:1 racemic mixture | 2-{[4-(2-acetamido-2-{[(4-ni...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15820 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 470 | -35.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15818 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15806 (1:1 racemic mixture | 2-{[4-(2-acetamido-2-{[(4-ni...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15821 (1:1 mixture of diastereomers | 2-[(4-{2-acetamido-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 540 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15821 (1:1 mixture of diastereomers | 2-[(4-{2-acetamido-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15811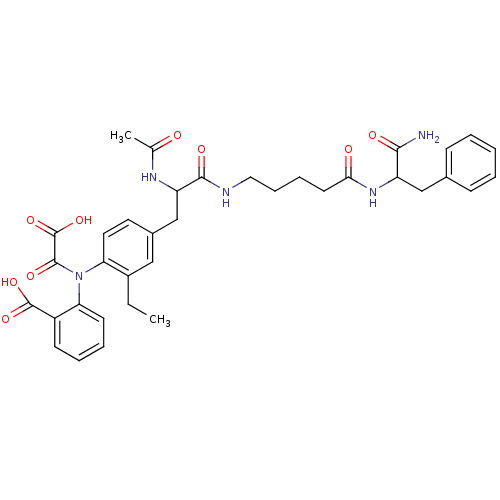 (2-({4-[2-({4-[(1-carbamoyl-2-phenylethyl)carbamoyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15816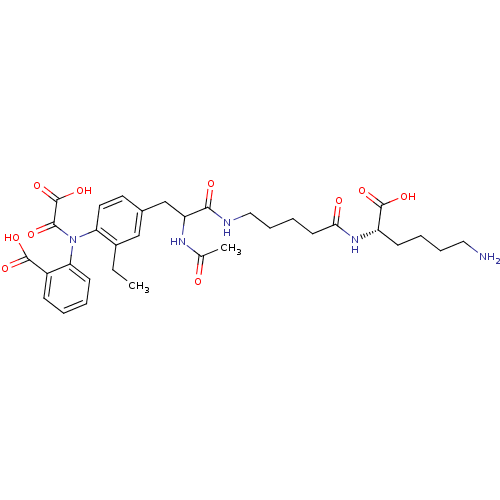 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 710 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15816 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13971 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15822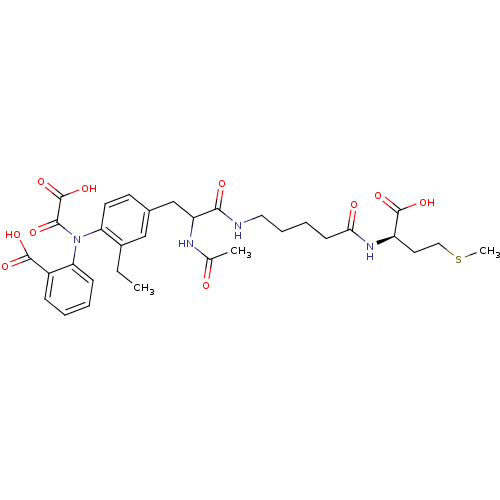 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1R)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15807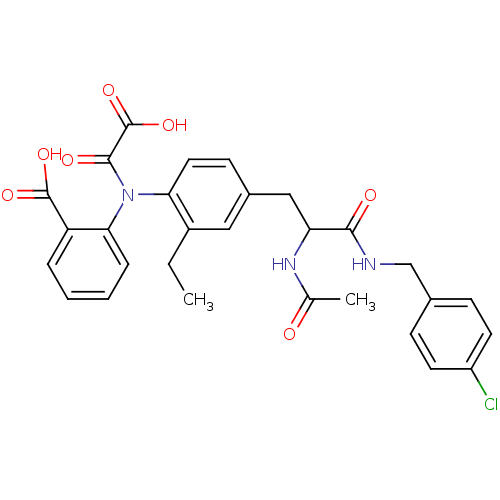 (1:1 racemic mixture | 2-{[4-(2-{[(4-chlorophenyl)m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13971 (1:1 racemic mixture | 2-({4-[2-acetamido-2-(pentyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15811 (2-({4-[2-({4-[(1-carbamoyl-2-phenylethyl)carbamoyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15810 (1:1 racemic mixture | 2-({4-[2-acetamido-2-({4-[(2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15807 (1:1 racemic mixture | 2-{[4-(2-{[(4-chlorophenyl)m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15822 (1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1R)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15809 (1:1 racemic mixture | 2-[(4-{2-[(4-carboxybutyl)ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15809 (1:1 racemic mixture | 2-[(4-{2-[(4-carboxybutyl)ca...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15810 (1:1 racemic mixture | 2-({4-[2-acetamido-2-({4-[(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | -30.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM15808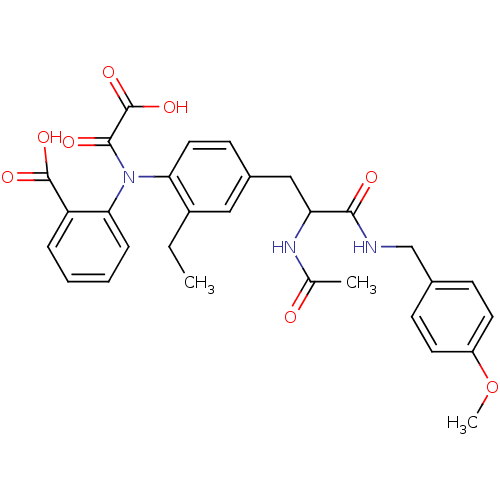 (1:1 racemic mixture | 2-{[4-(2-acetamido-2-{[(4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM15808 (1:1 racemic mixture | 2-{[4-(2-acetamido-2-{[(4-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13951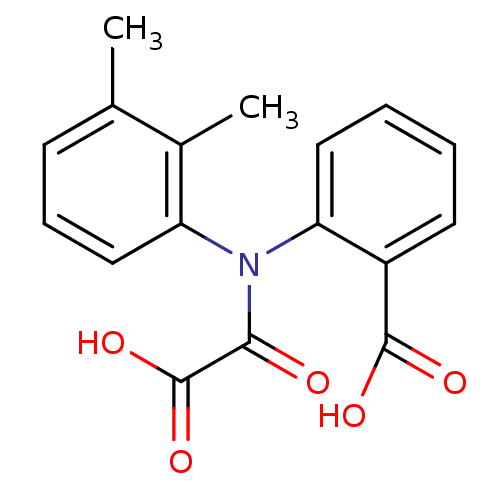 (2-[(2,3-Dimethylphenyl)oxalylamino]benzoic acid | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30E+4 | -22.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The phosphatase activity resulted in the formation of the colored product p-nitrophenol, which was continuously monitored at 405 nm every 30 s for 15... | Bioorg Med Chem Lett 13: 1887-90 (2003) Article DOI: 10.1016/S0960-894X(03)00302-0 BindingDB Entry DOI: 10.7270/Q29S1P91 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||