

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129484 (CHEMBL308050 | N-(2-Amino-ethyl)-2-[(S)-2-{3-[1-(2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129486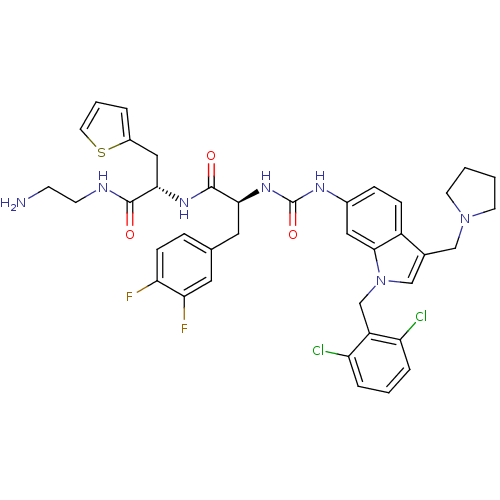 ((S)-N-[1-((S)-2-Amino-ethylcarbamoyl)-2-thiophen-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129487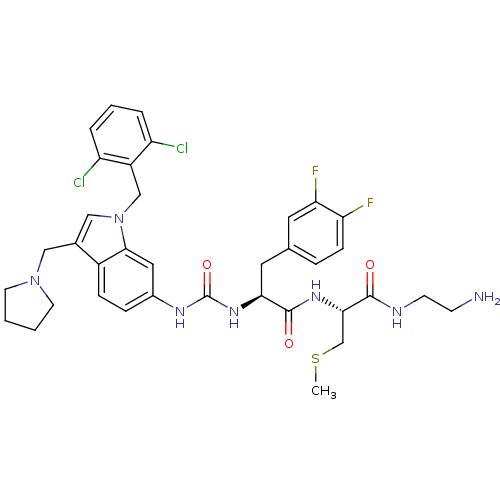 ((S)-N-[1-((R)-2-Amino-ethylcarbamoyl)-2-methylsulf...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129478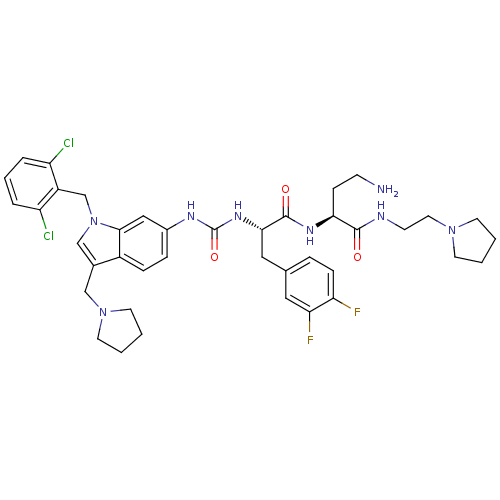 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129488 ((S)-N-[1-((S)-2-Amino-ethylcarbamoyl)-2-pyridin-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129495 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129493 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129491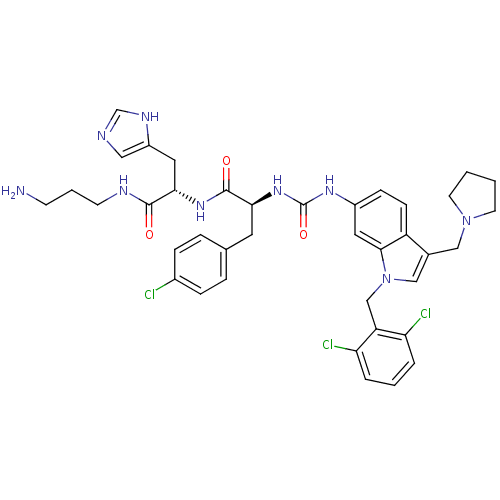 ((S)-N-[1-((S)-3-Amino-propylcarbamoyl)-2-(1H-imida...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129489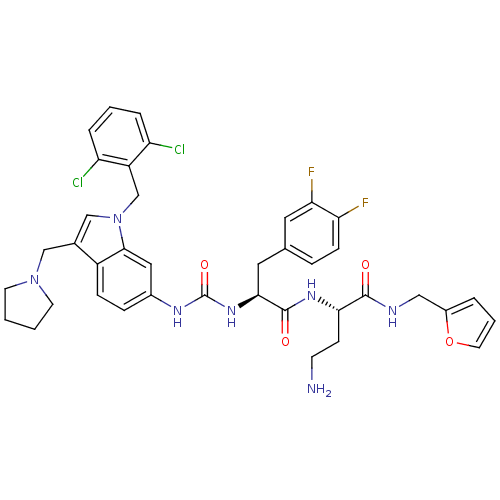 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129490 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129480 (5-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129485 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129492 (4-Amino-N-(3-amino-propyl)-2-((S)-3-(4-chloro-phen...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129481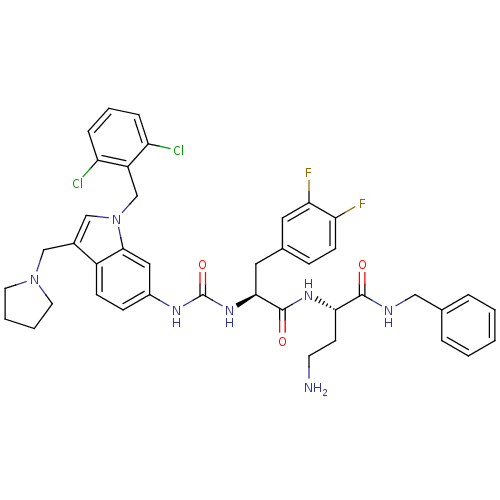 (4-Amino-N-benzyl-2-[(S)-2-{3-[1-(2,6-dichloro-benz...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129482 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129479 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129483 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteinase-activated receptor 1 (Homo sapiens (Human)) | BDBM50129494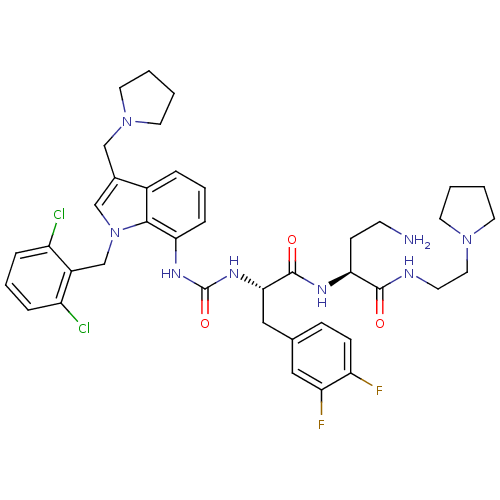 (4-Amino-2-[(S)-2-{3-[1-(2,6-dichloro-benzyl)-3-pyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]-S-(p-F-Phe)-homoarginine-L-homoarginine-KY-NH2 to thrombin receptor on the membranes of CHRF-288-11 cells | Bioorg Med Chem Lett 13: 2199-203 (2003) BindingDB Entry DOI: 10.7270/Q2NS0T8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||