 Found 55 hits of Enzyme Inhibition Constant Data
Found 55 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422472
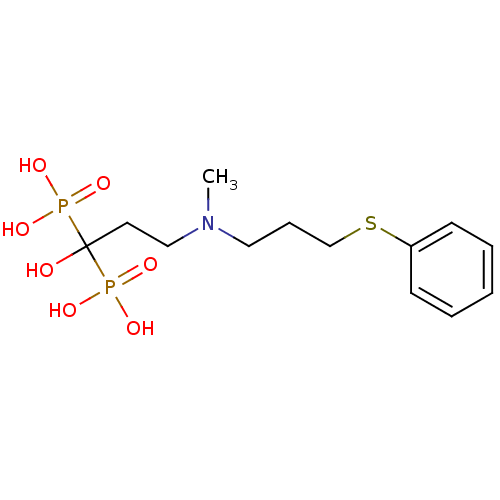
(CHEMBL100827)Show InChI InChI=1S/C13H23NO7P2S/c1-14(9-5-11-24-12-6-3-2-4-7-12)10-8-13(15,22(16,17)18)23(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25290
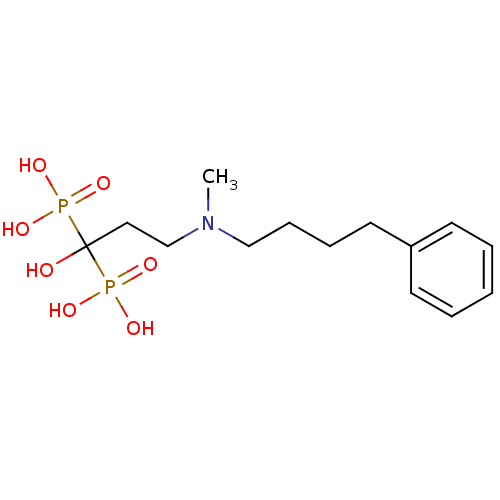
(CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...)Show InChI InChI=1S/C14H25NO7P2/c1-15(11-6-5-9-13-7-3-2-4-8-13)12-10-14(16,23(17,18)19)24(20,21)22/h2-4,7-8,16H,5-6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098389
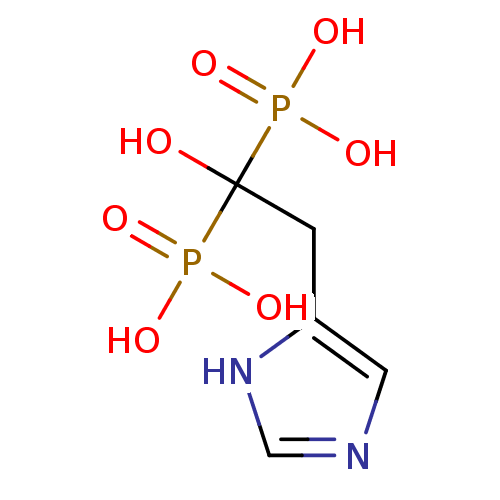
(1-hydroxy-2-(1H-imidazol-5-yl)ethane-1,1-diyldipho...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)1-4-2-6-3-7-4/h2-3,8H,1H2,(H,6,7)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422449

(CHEMBL101886)Show InChI InChI=1S/C13H23NO8P2/c1-14(9-5-11-22-12-6-3-2-4-7-12)10-8-13(15,23(16,17)18)24(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422469
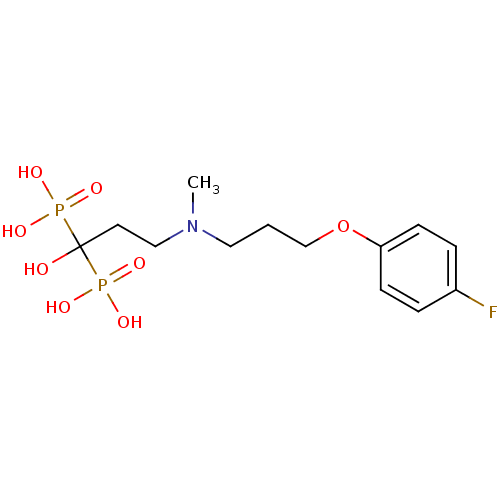
(CHEMBL101472)Show SMILES CN(CCCOc1ccc(F)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22FNO8P2/c1-15(8-2-10-23-12-5-3-11(14)4-6-12)9-7-13(16,24(17,18)19)25(20,21)22/h3-6,16H,2,7-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422457
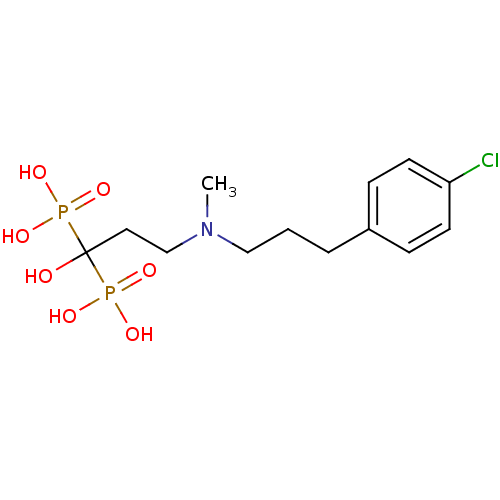
(CHEMBL101230)Show SMILES CN(CCCc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22ClNO7P2/c1-15(9-2-3-11-4-6-12(14)7-5-11)10-8-13(16,23(17,18)19)24(20,21)22/h4-7,16H,2-3,8-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422470
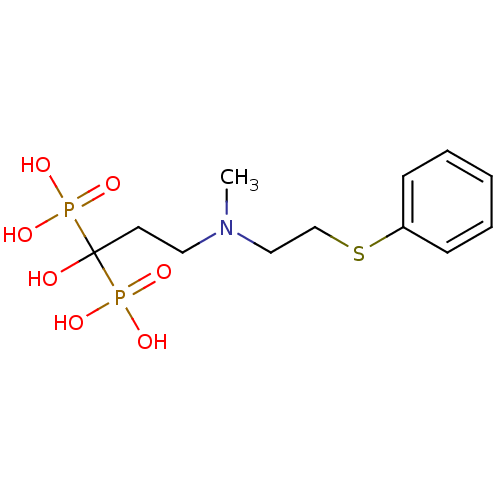
(CHEMBL100508)Show InChI InChI=1S/C12H21NO7P2S/c1-13(9-10-23-11-5-3-2-4-6-11)8-7-12(14,21(15,16)17)22(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422463
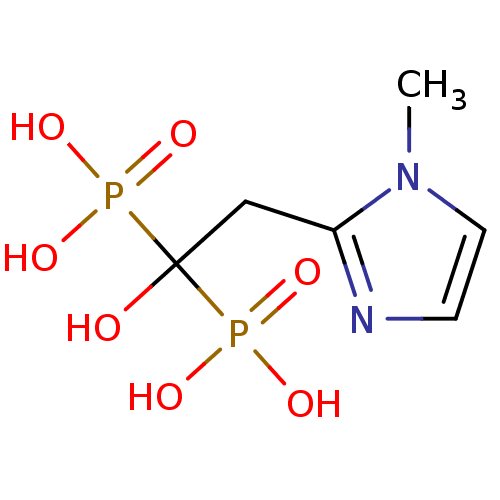
(CHEMBL101207)Show InChI InChI=1S/C6H12N2O7P2/c1-8-3-2-7-5(8)4-6(9,16(10,11)12)17(13,14)15/h2-3,9H,4H2,1H3,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50117257
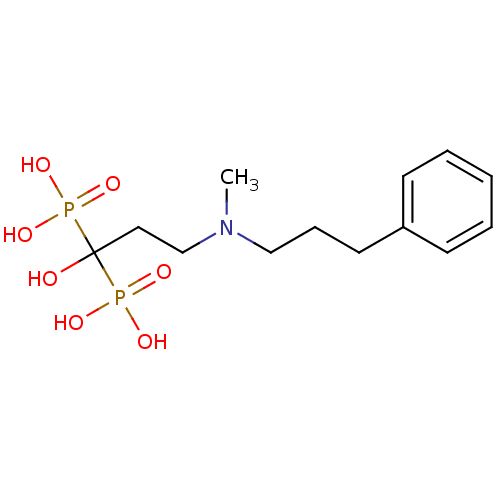
(1-hydroxy-3-(methyl(3-phenylpropyl)amino)propane-1...)Show InChI InChI=1S/C13H23NO7P2/c1-14(10-5-8-12-6-3-2-4-7-12)11-9-13(15,22(16,17)18)23(19,20)21/h2-4,6-7,15H,5,8-11H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135839
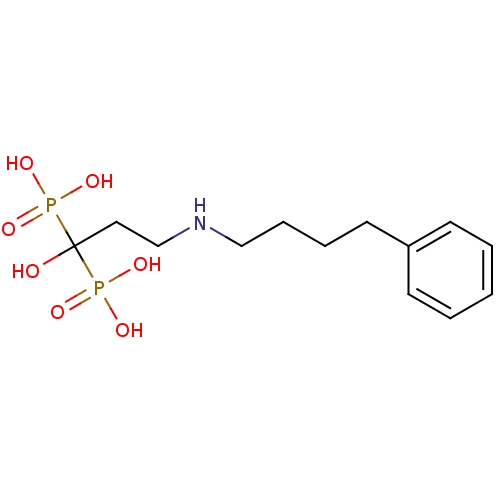
(CHEMBL55464 | [1-Hydroxy-3-(4-phenyl-butylamino)-1...)Show InChI InChI=1S/C13H23NO7P2/c15-13(22(16,17)18,23(19,20)21)9-11-14-10-5-4-8-12-6-2-1-3-7-12/h1-3,6-7,14-15H,4-5,8-11H2,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422455
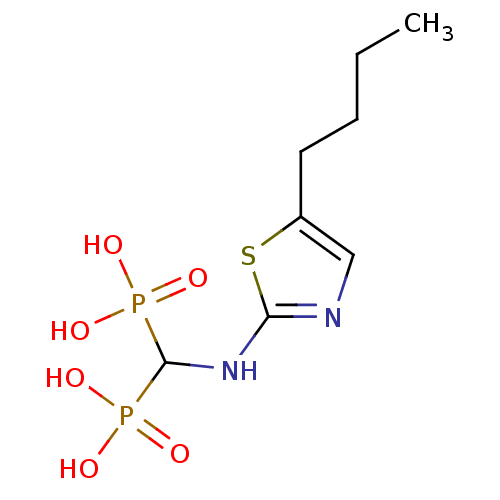
(CHEMBL98476)Show InChI InChI=1S/C8H16N2O6P2S/c1-2-3-4-6-5-9-7(19-6)10-8(17(11,12)13)18(14,15)16/h5,8H,2-4H2,1H3,(H,9,10)(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422454
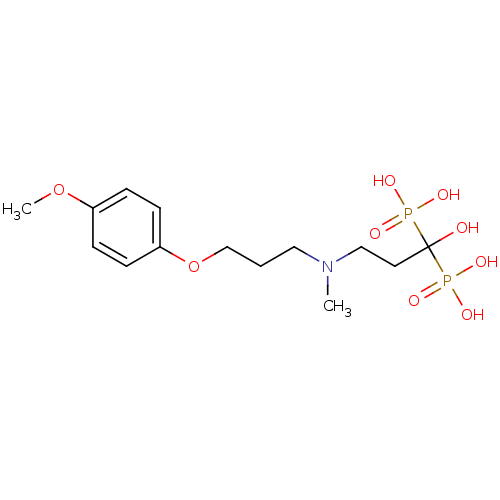
(CHEMBL316913)Show SMILES COc1ccc(OCCCN(C)CCC(O)(P(O)(O)=O)P(O)(O)=O)cc1 Show InChI InChI=1S/C14H25NO9P2/c1-15(10-8-14(16,25(17,18)19)26(20,21)22)9-3-11-24-13-6-4-12(23-2)5-7-13/h4-7,16H,3,8-11H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422446
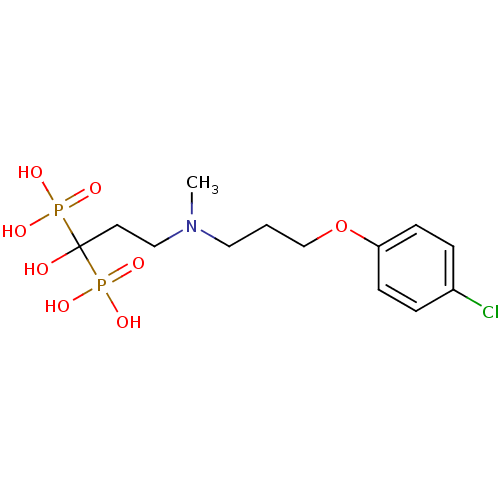
(CHEMBL101407)Show SMILES CN(CCCOc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22ClNO8P2/c1-15(8-2-10-23-12-5-3-11(14)4-6-12)9-7-13(16,24(17,18)19)25(20,21)22/h3-6,16H,2,7-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135831
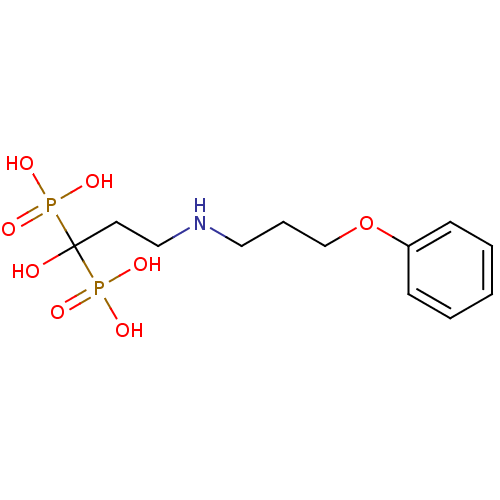
(CHEMBL316844 | [1-Hydroxy-3-(3-phenoxy-propylamino...)Show InChI InChI=1S/C12H21NO8P2/c14-12(22(15,16)17,23(18,19)20)7-9-13-8-4-10-21-11-5-2-1-3-6-11/h1-3,5-6,13-14H,4,7-10H2,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422448
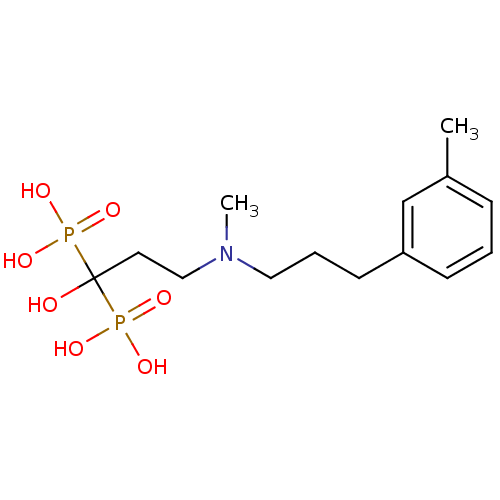
(CHEMBL100441)Show SMILES CN(CCCc1cccc(C)c1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO7P2/c1-12-5-3-6-13(11-12)7-4-9-15(2)10-8-14(16,23(17,18)19)24(20,21)22/h3,5-6,11,16H,4,7-10H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50117260
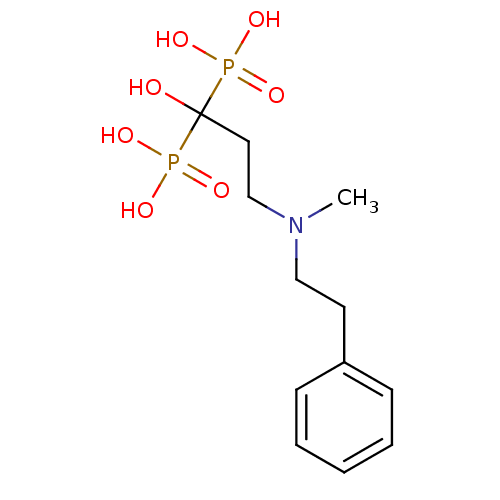
(1-hydroxy-3-(methyl(phenethyl)amino)propane-1,1-di...)Show InChI InChI=1S/C12H21NO7P2/c1-13(9-7-11-5-3-2-4-6-11)10-8-12(14,21(15,16)17)22(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138041
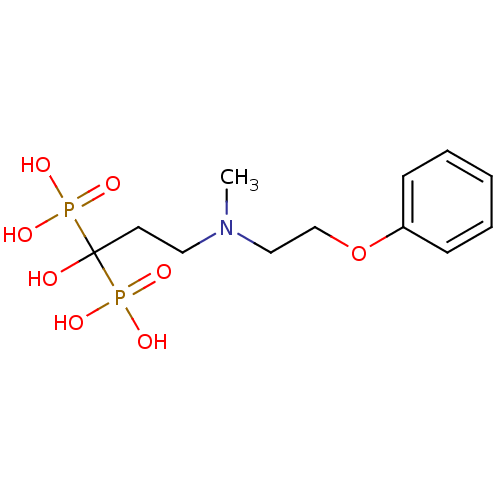
(1-hydroxy-3-(methyl(2-phenoxyethyl)amino)propane-1...)Show InChI InChI=1S/C12H21NO8P2/c1-13(9-10-21-11-5-3-2-4-6-11)8-7-12(14,22(15,16)17)23(18,19)20/h2-6,14H,7-10H2,1H3,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422464
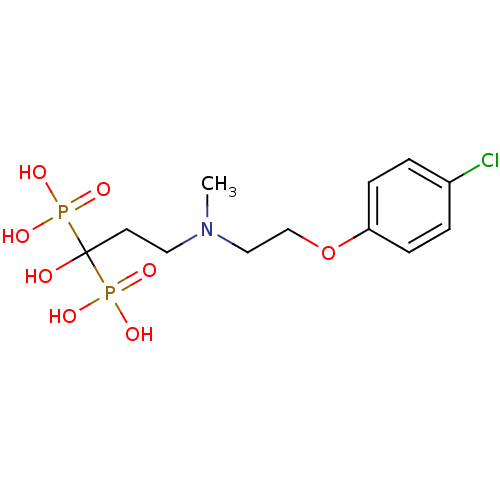
(CHEMBL319519)Show SMILES CN(CCOc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C12H20ClNO8P2/c1-14(8-9-22-11-4-2-10(13)3-5-11)7-6-12(15,23(16,17)18)24(19,20)21/h2-5,15H,6-9H2,1H3,(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422458
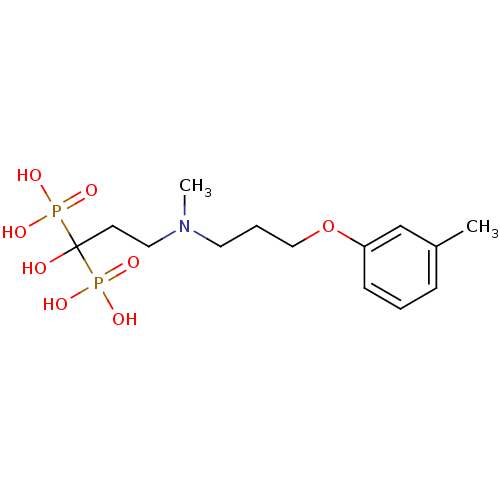
(CHEMBL100835)Show SMILES CN(CCCOc1cccc(C)c1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO8P2/c1-12-5-3-6-13(11-12)23-10-4-8-15(2)9-7-14(16,24(17,18)19)25(20,21)22/h3,5-6,11,16H,4,7-10H2,1-2H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422467
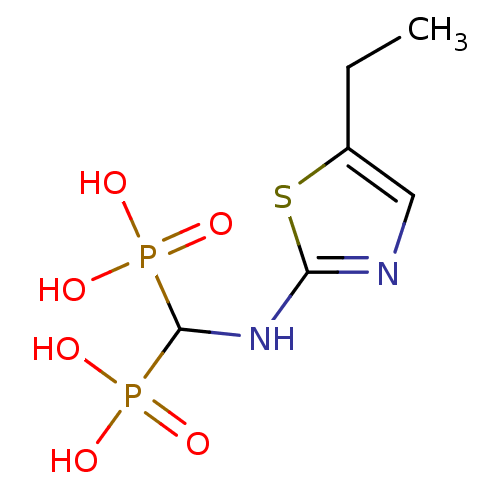
(CHEMBL100830)Show InChI InChI=1S/C6H12N2O6P2S/c1-2-4-3-7-5(17-4)8-6(15(9,10)11)16(12,13)14/h3,6H,2H2,1H3,(H,7,8)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422475
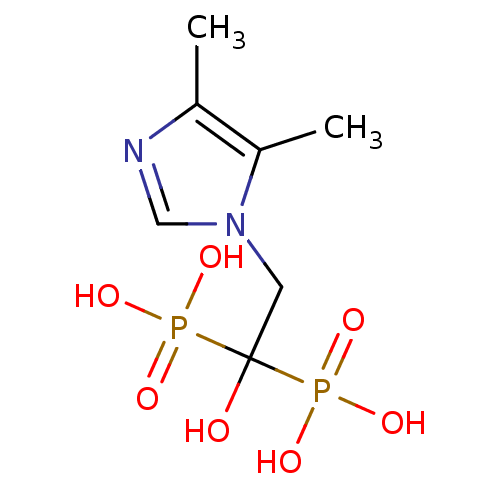
(CHEMBL98703)Show InChI InChI=1S/C7H14N2O7P2/c1-5-6(2)9(4-8-5)3-7(10,17(11,12)13)18(14,15)16/h4,10H,3H2,1-2H3,(H2,11,12,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422477
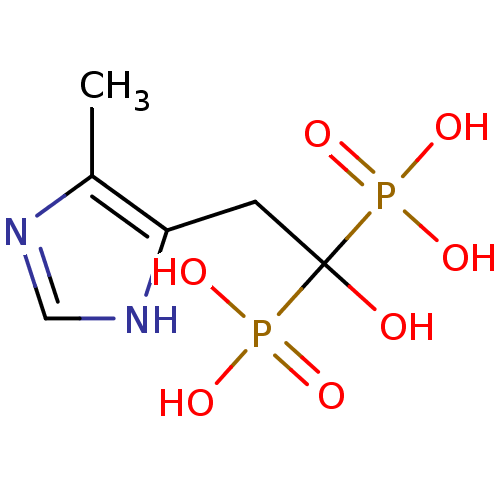
(CHEMBL317646)Show InChI InChI=1S/C6H12N2O7P2/c1-4-5(8-3-7-4)2-6(9,16(10,11)12)17(13,14)15/h3,9H,2H2,1H3,(H,7,8)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
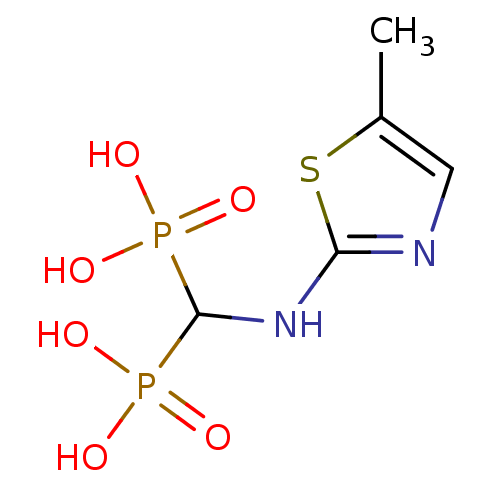
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422461
(CHEMBL100335)Show InChI InChI=1S/C5H10N2O6P2S/c1-3-2-6-4(16-3)7-5(14(8,9)10)15(11,12)13/h2,5H,1H3,(H,6,7)(H2,8,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422444

(CHEMBL100295)Show InChI InChI=1S/C7H14N2O6P2S/c1-2-3-5-4-8-6(18-5)9-7(16(10,11)12)17(13,14)15/h4,7H,2-3H2,1H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
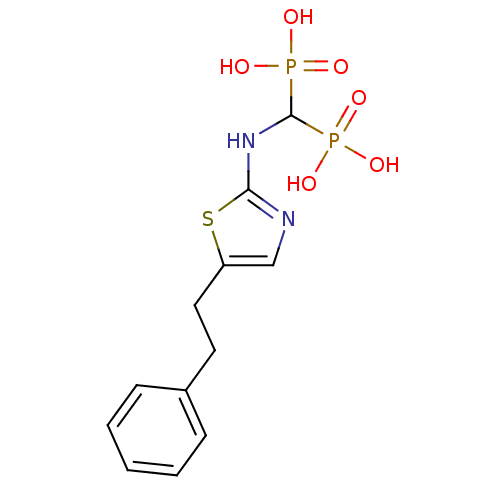
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422441
(CHEMBL101539)Show InChI InChI=1S/C12H16N2O6P2S/c15-21(16,17)12(22(18,19)20)14-11-13-8-10(23-11)7-6-9-4-2-1-3-5-9/h1-5,8,12H,6-7H2,(H,13,14)(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
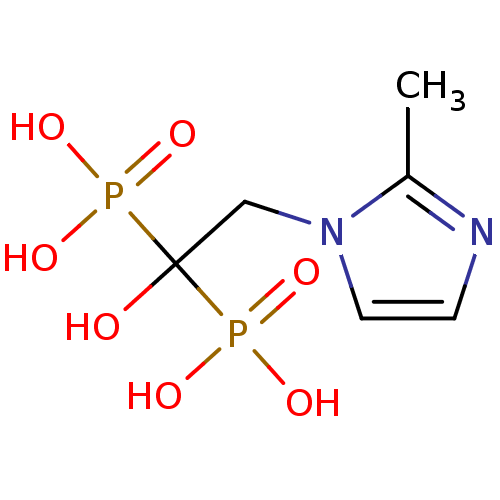
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422445
(CHEMBL318071)Show InChI InChI=1S/C6H12N2O7P2/c1-5-7-2-3-8(5)4-6(9,16(10,11)12)17(13,14)15/h2-3,9H,4H2,1H3,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422450
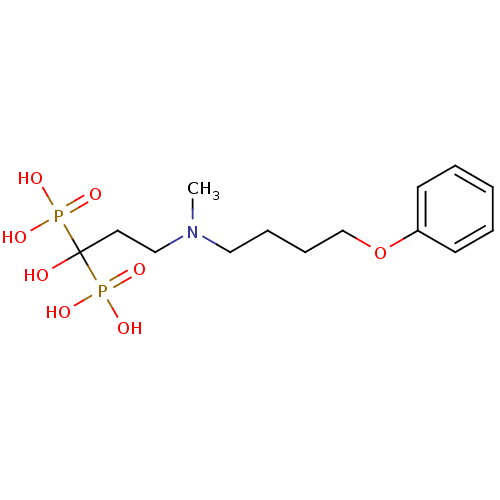
(CHEMBL316663)Show SMILES CN(CCCCOc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO8P2/c1-15(10-5-6-12-23-13-7-3-2-4-8-13)11-9-14(16,24(17,18)19)25(20,21)22/h2-4,7-8,16H,5-6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422436

(CHEMBL97911)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-4-6-1-2-7-4/h1-2,8H,3H2,(H,6,7)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422471
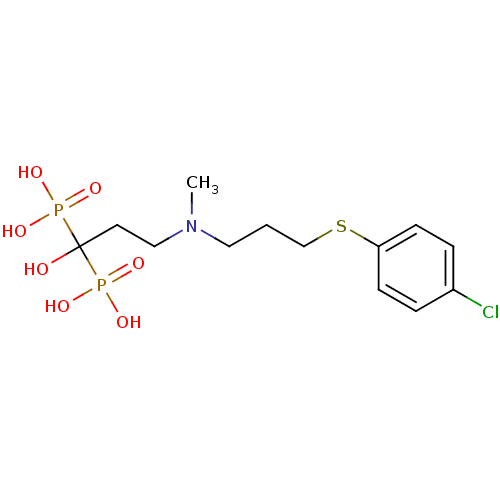
(CHEMBL102209)Show SMILES CN(CCCSc1ccc(Cl)cc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C13H22ClNO7P2S/c1-15(8-2-10-25-12-5-3-11(14)4-6-12)9-7-13(16,23(17,18)19)24(20,21)22/h3-6,16H,2,7-10H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422478

(CHEMBL100831)Show InChI InChI=1S/C4H8N2O6P2S/c7-13(8,9)4(14(10,11)12)6-3-5-1-2-15-3/h1-2,4H,(H,5,6)(H2,7,8,9)(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422453

(CHEMBL323300)Show InChI InChI=1S/C5H11N3O6P2/c1-8-3-2-6-4(8)7-5(15(9,10)11)16(12,13)14/h2-3,5H,1H3,(H,6,7)(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422456

(CHEMBL100232)Show InChI InChI=1S/C12H21NO7P2S/c14-12(21(15,16)17,22(18,19)20)7-9-13-8-4-10-23-11-5-2-1-3-6-11/h1-3,5-6,13-14H,4,7-10H2,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422481

(CHEMBL318861)Show SMILES CCCN(CCCOc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C15H27NO8P2/c1-2-10-16(11-6-13-24-14-7-4-3-5-8-14)12-9-15(17,25(18,19)20)26(21,22)23/h3-5,7-8,17H,2,6,9-13H2,1H3,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422474

(CHEMBL321075)Show InChI InChI=1S/C14H25NO7P2/c1-2-15(11-6-9-13-7-4-3-5-8-13)12-10-14(16,23(17,18)19)24(20,21)22/h3-5,7-8,16H,2,6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422439

(CHEMBL102185)Show SMILES CN(CCCCCc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C15H27NO7P2/c1-16(12-7-3-6-10-14-8-4-2-5-9-14)13-11-15(17,24(18,19)20)25(21,22)23/h2,4-5,8-9,17H,3,6-7,10-13H2,1H3,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422442
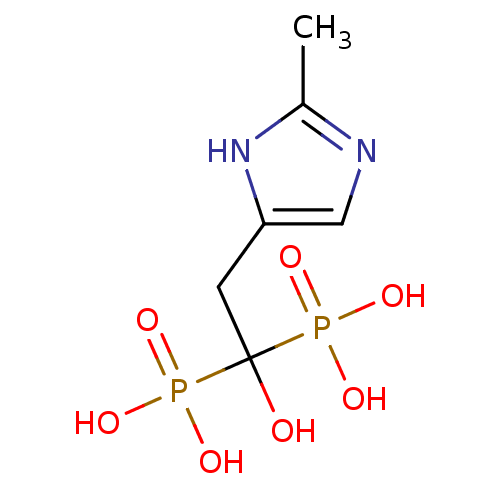
(CHEMBL323299)Show InChI InChI=1S/C6H12N2O7P2/c1-4-7-3-5(8-4)2-6(9,16(10,11)12)17(13,14)15/h3,9H,2H2,1H3,(H,7,8)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422437

(CHEMBL273286)Show InChI InChI=1S/C12H21NO7P2/c14-12(21(15,16)17,22(18,19)20)8-10-13-9-4-7-11-5-2-1-3-6-11/h1-3,5-6,13-14H,4,7-10H2,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422468

(CHEMBL98787)Show SMILES OC(Cc1nccn1C(=O)c1ccccc1)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C12H14N2O8P2/c15-11(9-4-2-1-3-5-9)14-7-6-13-10(14)8-12(16,23(17,18)19)24(20,21)22/h1-7,16H,8H2,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422462
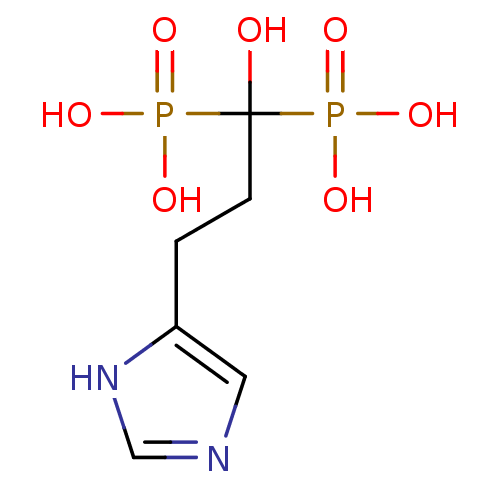
(CHEMBL98211)Show InChI InChI=1S/C6H12N2O7P2/c9-6(16(10,11)12,17(13,14)15)2-1-5-3-7-4-8-5/h3-4,9H,1-2H2,(H,7,8)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422459

(CHEMBL101717)Show SMILES CCN(CCCOc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO8P2/c1-2-15(10-6-12-23-13-7-4-3-5-8-13)11-9-14(16,24(17,18)19)25(20,21)22/h3-5,7-8,16H,2,6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422480

(CHEMBL101647)Show InChI InChI=1S/C6H12N2O7P2/c9-6(16(10,11)12,17(13,14)15)1-3-8-4-2-7-5-8/h2,4-5,9H,1,3H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 157 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422466

(CHEMBL318504)Show InChI InChI=1S/C11H15N3O6P2/c15-21(16,17)11(22(18,19)20)13-10-12-6-7-14(10)8-9-4-2-1-3-5-9/h1-7,11H,8H2,(H,12,13)(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 185 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422447

(CHEMBL101716)Show SMILES CN(CCCOc1ccccc1)CCCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C14H25NO8P2/c1-15(11-6-12-23-13-7-3-2-4-8-13)10-5-9-14(16,24(17,18)19)25(20,21)22/h2-4,7-8,16H,5-6,9-12H2,1H3,(H2,17,18,19)(H2,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 246 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50135841

((4-methylthiazol-2-ylamino)methylenediphosphonic a...)Show InChI InChI=1S/C5H10N2O6P2S/c1-3-2-16-4(6-3)7-5(14(8,9)10)15(11,12)13/h2,5H,1H3,(H,6,7)(H2,8,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 347 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422443

(CHEMBL327756)Show InChI InChI=1S/C10H13N3O6P2/c14-20(15,16)10(21(17,18)19)12-9-11-6-7-13(9)8-4-2-1-3-5-8/h1-7,10H,(H,11,12)(H2,14,15,16)(H2,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422440

(CHEMBL98080)Show InChI InChI=1S/C7H14N2O6P2S/c1-4(2)5-3-8-6(18-5)9-7(16(10,11)12)17(13,14)15/h3-4,7H,1-2H3,(H,8,9)(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 632 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422460
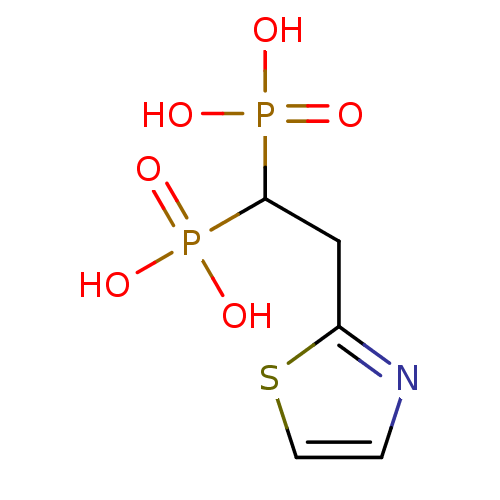
(CHEMBL98629)Show InChI InChI=1S/C5H9NO6P2S/c7-13(8,9)5(14(10,11)12)3-4-6-1-2-15-4/h1-2,5H,3H2,(H2,7,8,9)(H2,10,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 733 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422451

(CHEMBL98083)Show InChI InChI=1S/C11H19NO7P2/c13-11(20(14,15)16,21(17,18)19)7-9-12-8-6-10-4-2-1-3-5-10/h1-5,12-13H,6-9H2,(H2,14,15,16)(H2,17,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 885 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422465

(CHEMBL97920)Show SMILES CCCCN(CCCOc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C16H29NO8P2/c1-2-3-11-17(12-7-14-25-15-8-5-4-6-9-15)13-10-16(18,26(19,20)21)27(22,23)24/h4-6,8-9,18H,2-3,7,10-14H2,1H3,(H2,19,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422479

(CHEMBL100717)Show InChI InChI=1S/C4H9N3O6P2/c8-14(9,10)4(15(11,12)13)7-3-5-1-2-6-3/h1-2,4H,(H2,5,6,7)(H2,8,9,10)(H2,11,12,13) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422473
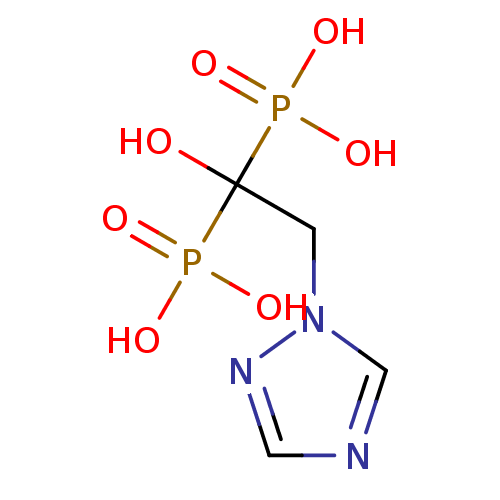
(CHEMBL99006)Show InChI InChI=1S/C4H9N3O7P2/c8-4(15(9,10)11,16(12,13)14)1-7-3-5-2-6-7/h2-3,8H,1H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422476
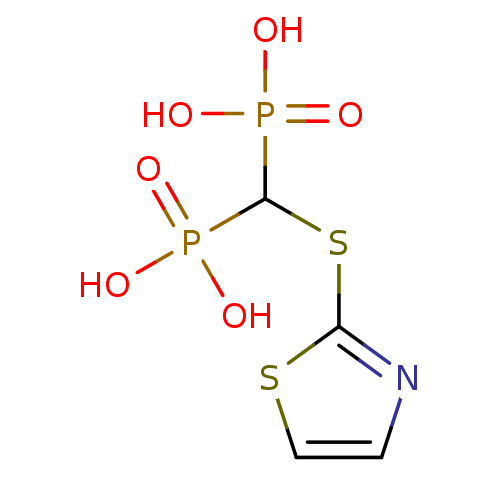
(CHEMBL101987)Show InChI InChI=1S/C4H7NO6P2S2/c6-12(7,8)4(13(9,10)11)15-3-5-1-2-14-3/h1-2,4H,(H2,6,7,8)(H2,9,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
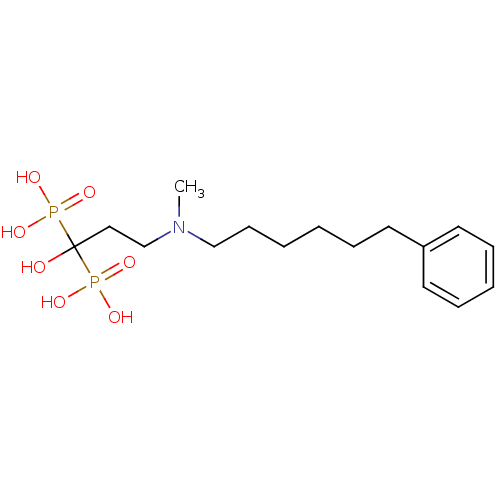
(Homo sapiens (Human)) | BDBM50422452
(CHEMBL317338)Show SMILES CN(CCCCCCc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C16H29NO7P2/c1-17(14-12-16(18,25(19,20)21)26(22,23)24)13-8-3-2-5-9-15-10-6-4-7-11-15/h4,6-7,10-11,18H,2-3,5,8-9,12-14H2,1H3,(H2,19,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50422438
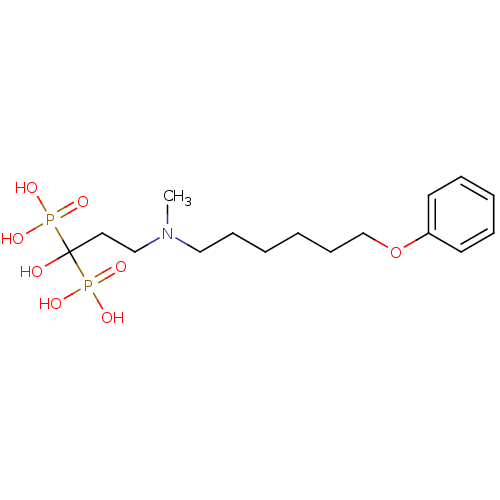
(CHEMBL432388)Show SMILES CN(CCCCCCOc1ccccc1)CCC(O)(P(O)(O)=O)P(O)(O)=O Show InChI InChI=1S/C16H29NO8P2/c1-17(13-11-16(18,26(19,20)21)27(22,23)24)12-7-2-3-8-14-25-15-9-5-4-6-10-15/h4-6,9-10,18H,2-3,7-8,11-14H2,1H3,(H2,19,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign
Curated by ChEMBL
| Assay Description
Negative logarithm of inhibitory concentration against bone resorption |
J Med Chem 46: 2932-44 (2003)
Article DOI: 10.1021/jm030054u
BindingDB Entry DOI: 10.7270/Q2R78GHD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data