 Found 28 hits of Enzyme Inhibition Constant Data
Found 28 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 (MMP-3) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-9 (MMP-9) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 (MMP-2) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloprotease-1 (MMP-1) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131664

(CHEMBL326489 | N*4*-Hydroxy-N*1*-{1-[(methoxy-phen...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H43N3O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(38)37-41)31(39)35-30(34(2,3)4)32(40)36-33(42-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33,41H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 (MMP-3) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-14 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-13 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 (MMP-3) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104969
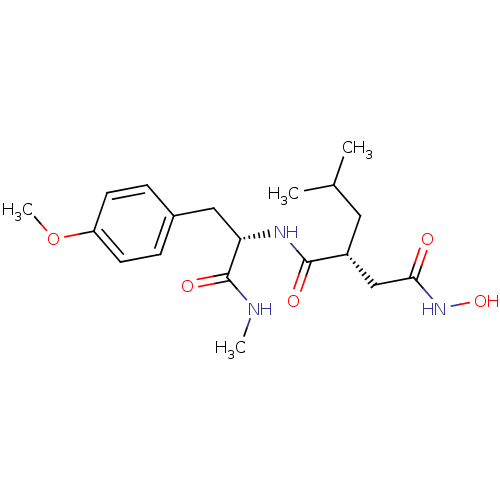
((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 (MMP-2) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Tumor necrosis factor alpha converting enzyme (TACE) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloprotease-1 (MMP-1) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131663

(3-{1-[(Methoxy-phenyl-methyl)-carbamoyl]-2,2-dimet...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H42N2O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(37)38)31(39)35-30(34(2,3)4)32(40)36-33(41-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 (MMP-3) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-7 (MMP-7) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50104969

((R)-N*4*-Hydroxy-2-isobutyl-N*1*-[(S)-2-(4-methoxy...)Show SMILES CNC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)[C@H](CC(C)C)CC(=O)NO Show InChI InChI=1S/C19H29N3O5/c1-12(2)9-14(11-17(23)22-26)18(24)21-16(19(25)20-3)10-13-5-7-15(27-4)8-6-13/h5-8,12,14,16,26H,9-11H2,1-4H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-3 (MMP-3) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-13 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131664

(CHEMBL326489 | N*4*-Hydroxy-N*1*-{1-[(methoxy-phen...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H43N3O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(38)37-41)31(39)35-30(34(2,3)4)32(40)36-33(42-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33,41H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-9 (MMP-9) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131664

(CHEMBL326489 | N*4*-Hydroxy-N*1*-{1-[(methoxy-phen...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H43N3O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(38)37-41)31(39)35-30(34(2,3)4)32(40)36-33(42-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33,41H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 (MMP-2) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 840 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-9 (MMP-9) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50131664

(CHEMBL326489 | N*4*-Hydroxy-N*1*-{1-[(methoxy-phen...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H43N3O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(38)37-41)31(39)35-30(34(2,3)4)32(40)36-33(42-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33,41H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-14 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 (MMP-2) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-14 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131663

(3-{1-[(Methoxy-phenyl-methyl)-carbamoyl]-2,2-dimet...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H42N2O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(37)38)31(39)35-30(34(2,3)4)32(40)36-33(41-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-13 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50131664

(CHEMBL326489 | N*4*-Hydroxy-N*1*-{1-[(methoxy-phen...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H43N3O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(38)37-41)31(39)35-30(34(2,3)4)32(40)36-33(42-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33,41H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloprotease-1 (MMP-1) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131663

(3-{1-[(Methoxy-phenyl-methyl)-carbamoyl]-2,2-dimet...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H42N2O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(37)38)31(39)35-30(34(2,3)4)32(40)36-33(41-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-9 (MMP-9) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131663

(3-{1-[(Methoxy-phenyl-methyl)-carbamoyl]-2,2-dimet...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H42N2O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(37)38)31(39)35-30(34(2,3)4)32(40)36-33(41-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human matrix metalloprotease-2 (MMP-2) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Matrix metalloprotease-1 (MMP-1) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50097263

((R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylca...)Show SMILES C[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(=O)NO)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C34H43N3O4/c1-23-21-25(19-20-29(23)27-16-10-7-11-17-27)13-12-18-28(22-30(38)37-41)32(39)36-31(34(3,4)5)33(40)35-24(2)26-14-8-6-9-15-26/h6-11,14-17,19-21,24,28,31,41H,12-13,18,22H2,1-5H3,(H,35,40)(H,36,39)(H,37,38)/t24-,28-,31-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against Procollagen C-terminal proteinase (PCP) |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50131663

(3-{1-[(Methoxy-phenyl-methyl)-carbamoyl]-2,2-dimet...)Show SMILES CO[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CCCc1ccc(c(C)c1)-c1ccccc1)CC(O)=O)C(C)(C)C)c1ccccc1 Show InChI InChI=1S/C34H42N2O5/c1-23-21-24(19-20-28(23)25-14-8-6-9-15-25)13-12-18-27(22-29(37)38)31(39)35-30(34(2,3)4)32(40)36-33(41-5)26-16-10-7-11-17-26/h6-11,14-17,19-21,27,30,33H,12-13,18,22H2,1-5H3,(H,35,39)(H,36,40)(H,37,38)/t27-,30-,33-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-14 |
J Med Chem 46: 3514-25 (2003)
Article DOI: 10.1021/jm0308038
BindingDB Entry DOI: 10.7270/Q24F1Q3R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data