

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000806 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000806 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000799 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the percentage of maximum inhibition at Opioid receptor delta 1 | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000807 (13-[2-Amino-3-(4-hydroxy-3-methoxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000807 (13-[2-Amino-3-(4-hydroxy-3-methoxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000800 (13-[2-Amino-3-(4-hydroxy-3-iodo-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000799 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000808 (13-[2-Amino-3-(3-amino-4-hydroxy-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000808 (13-[2-Amino-3-(3-amino-4-hydroxy-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against delta opioid receptors of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000807 (13-[2-Amino-3-(4-hydroxy-3-methoxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000802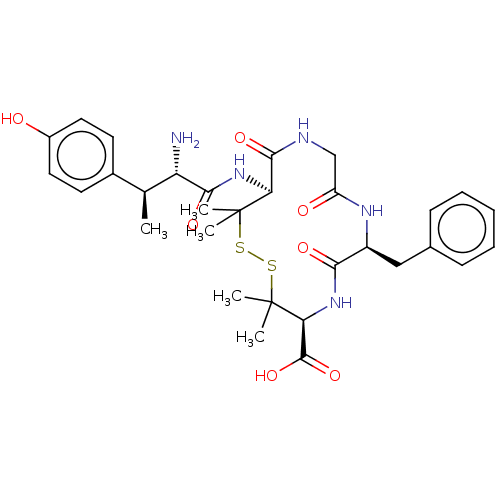 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 243 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the percentage of maximum inhibition at Opioid receptor delta 1 | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000800 (13-[2-Amino-3-(4-hydroxy-3-iodo-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 262 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000809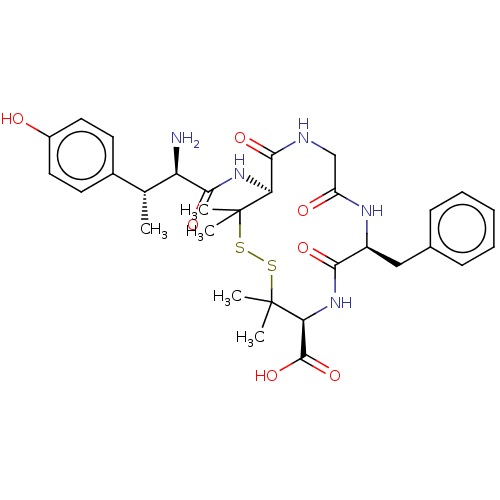 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 426 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000806 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000802 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 609 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (opioid receptor mu selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000803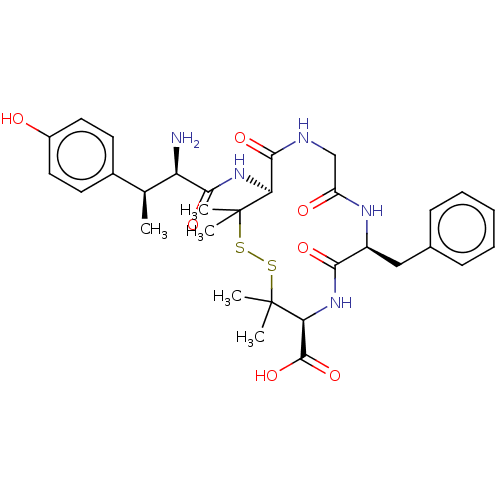 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 698 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the percentage of maximum inhibition at Opioid receptor delta 1 | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000804 (13-[2-Amino-3-(4-hydroxy-3-nitro-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000801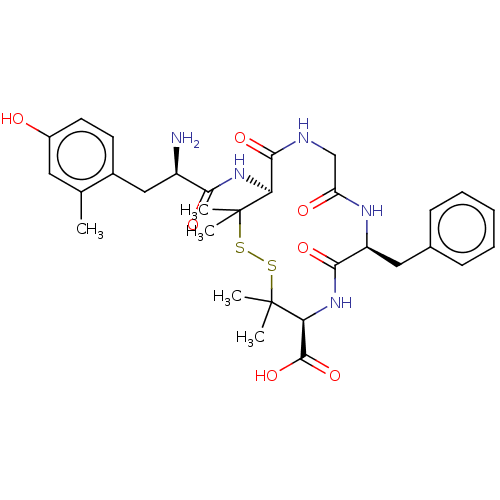 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 763 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the percentage of maximum inhibition at Opioid receptor delta 1 | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000804 (13-[2-Amino-3-(4-hydroxy-3-nitro-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000801 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000806 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (opioid receptor mu selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (opioid receptor mu selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (opioid receptor mu selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000804 (13-[2-Amino-3-(4-hydroxy-3-nitro-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (opioid receptor mu selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000799 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000807 (13-[2-Amino-3-(4-hydroxy-3-methoxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000804 (13-[2-Amino-3-(4-hydroxy-3-nitro-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]- DPDPE (opioid receptor delta selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000800 (13-[2-Amino-3-(4-hydroxy-3-iodo-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000805 (13-[(2-Amino-6-hydroxy-1,2,3,4,4a,8a-hexahydro-nap...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000801 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000802 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001683 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000808 (13-[2-Amino-3-(3-amino-4-hydroxy-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000801 (13-[2-Amino-3-(4-hydroxy-2-methyl-phenyl)-propiony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000802 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000803 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for the binding affinity in comparison with [3H]-[p-Cl-Phe4]-DPDPE (delta opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000799 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50000809 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Mouse vas deferens | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000809 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000800 (13-[2-Amino-3-(4-hydroxy-3-iodo-phenyl)-propionyla...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against delta opioid receptors of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50000803 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its ability to displace [3H]- CTOP (mu opioid receptor selective ligand) | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000803 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000804 (13-[2-Amino-3-(4-hydroxy-3-nitro-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against opioid receptor delta of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000808 (13-[2-Amino-3-(3-amino-4-hydroxy-phenyl)-propionyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000809 (13-[2-Amino-3-(4-hydroxy-phenyl)-butyrylamino]-7-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000807 (13-[2-Amino-3-(4-hydroxy-3-methoxy-phenyl)-propion...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory potency against Opioid receptor delta 1 of Guinea pig ileum | J Med Chem 35: 2384-91 (1992) BindingDB Entry DOI: 10.7270/Q2DF6Q4K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||