

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM13976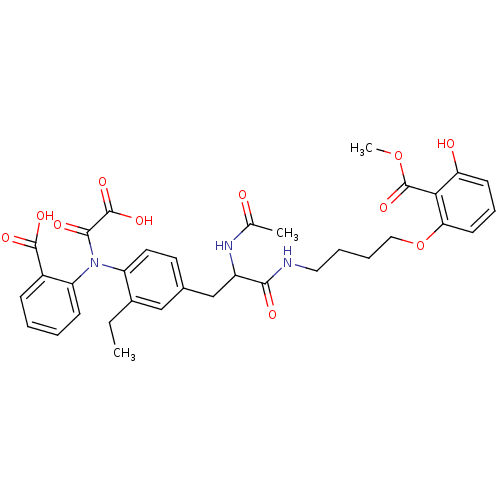 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of the compound was determined against Protein-tyrosine phosphatase 1B (PTB1B) | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM13976 (Aminobenzoic acid analog 5 | CHEMBL116605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50133280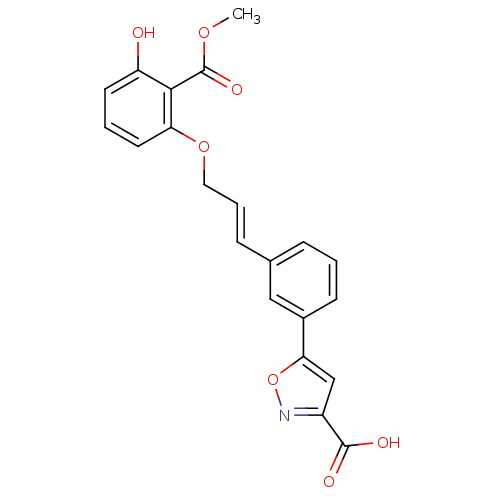 (5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against protein tyrosine phosphatase PTB1B | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50133279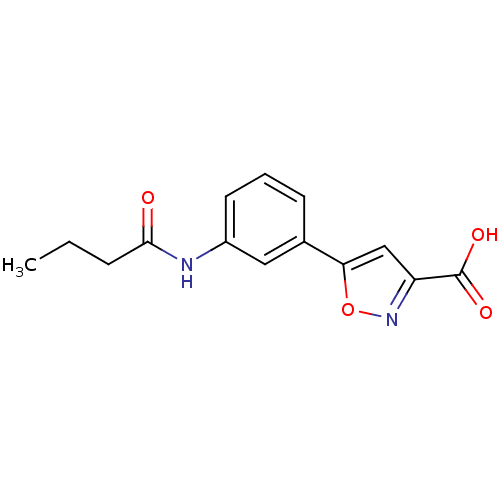 (5-(3-Butyrylamino-phenyl)-isoxazole-3-carboxylic a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.48E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against protein tyrosine phosphatase PTB1B | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 2 (Homo sapiens (Human)) | BDBM13990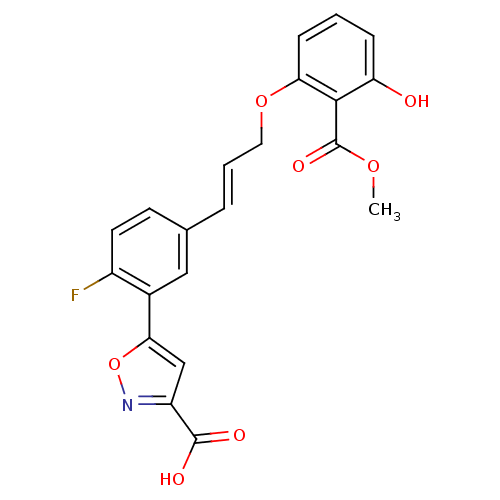 (5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity of the compound was determined against T cell protein tyrosine phosphatase (TCPTP) | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50133280 (5-(3-(3-(3-hydroxy-2-(methoxycarbonyl)phenoxy)prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase alpha (Homo sapiens (Human)) | BDBM50133279 (5-(3-Butyrylamino-phenyl)-isoxazole-3-carboxylic a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory constant of compound against T cell protein tyrosine phosphatase was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM48319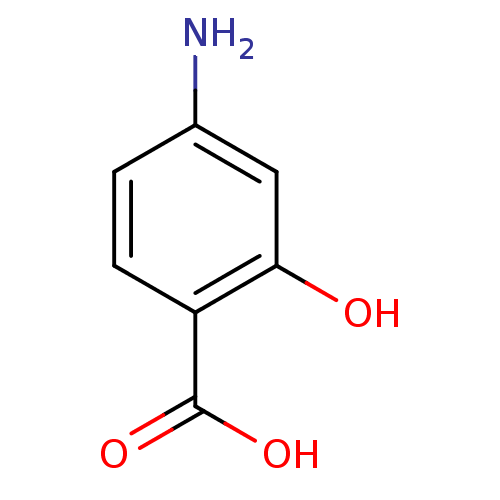 (4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Dissociation constant of the compound against protein tyrosine phosphatase PTB1B receptor was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Homo sapiens (Human)) | BDBM50133281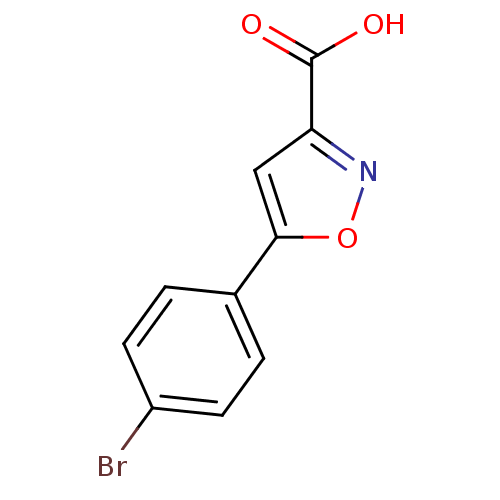 (5-(4-Bromo-phenyl)-isoxazole-3-carboxylic acid | C...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 8.00E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Dissociation constant of the compound against Protein-tyrosine phosphatase 1B receptor was determined | J Med Chem 46: 4232-5 (2003) Article DOI: 10.1021/jm034122o BindingDB Entry DOI: 10.7270/Q2BP0264 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||