

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003252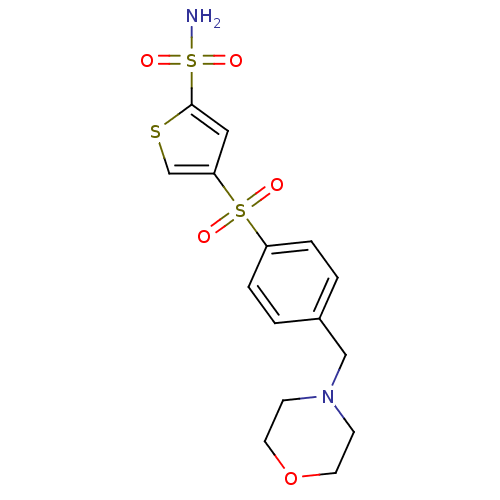 (4-(4-Morpholin-4-ylmethyl-benzenesulfonyl)-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003246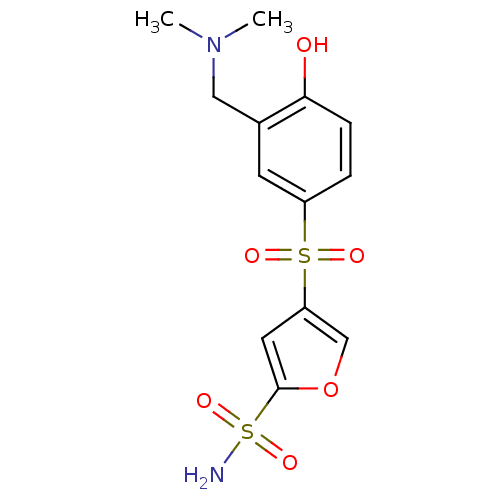 (4-(3-Dimethylaminomethyl-4-hydroxy-benzenesulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003246 (4-(3-Dimethylaminomethyl-4-hydroxy-benzenesulfonyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003247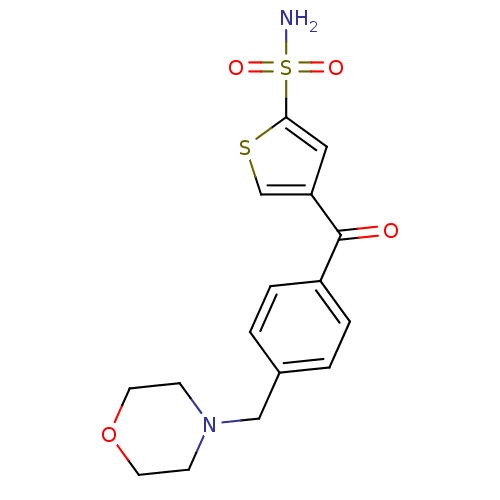 (4-(4-Morpholin-4-ylmethyl-benzoyl)-thiophene-2-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003244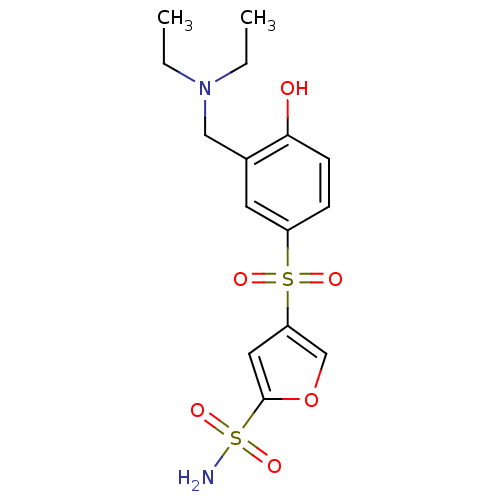 (4-(3-Diethylaminomethyl-4-hydroxy-benzenesulfonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003245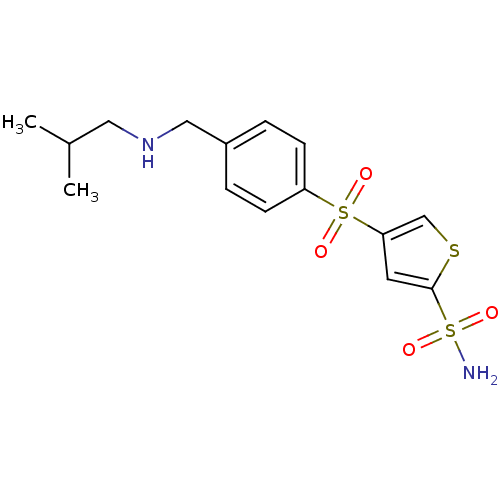 (4-[4-(Isobutylamino-methyl)-benzenesulfonyl]-thiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003251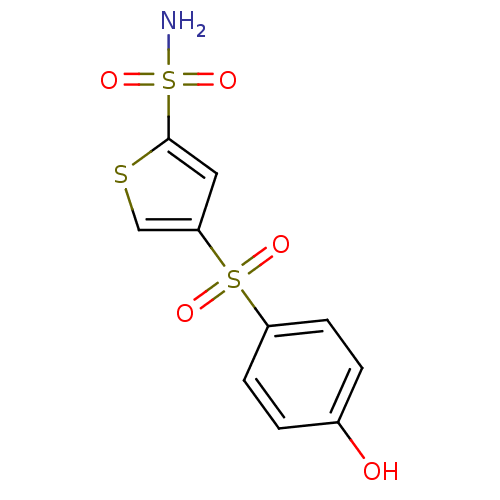 (4-(4-Hydroxy-benzenesulfonyl)-thiophene-2-sulfonic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003243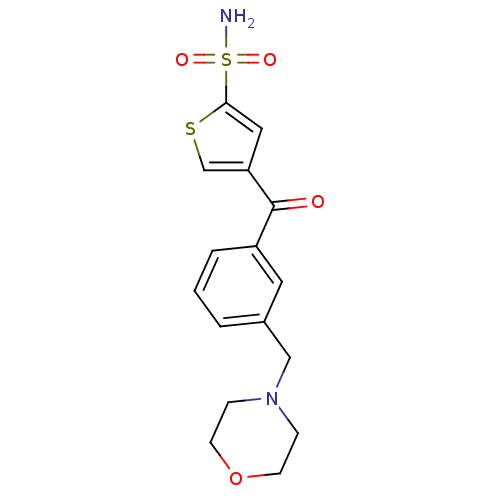 (4-(3-Morpholin-4-ylmethyl-benzoyl)-thiophene-2-sul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003242 (4-[3-(Isobutylamino-methyl)-benzoyl]-thiophene-2-s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003248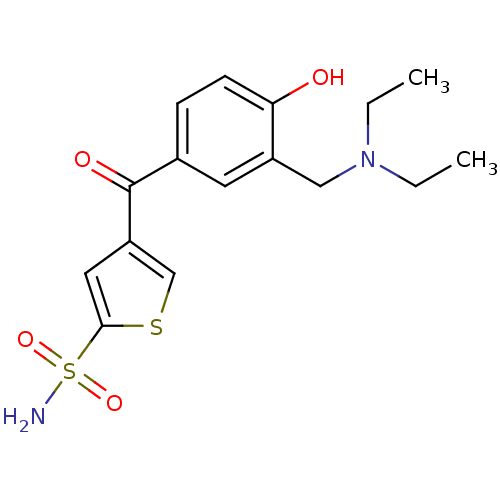 (4-(3-Diethylaminomethyl-4-hydroxy-benzoyl)-thiophe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003249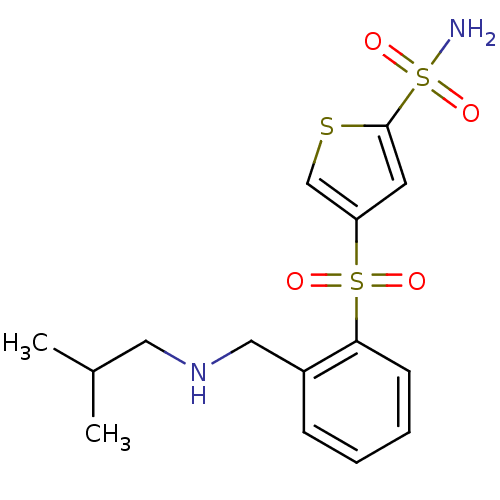 (4-[2-(Isobutylamino-methyl)-benzenesulfonyl]-thiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50003250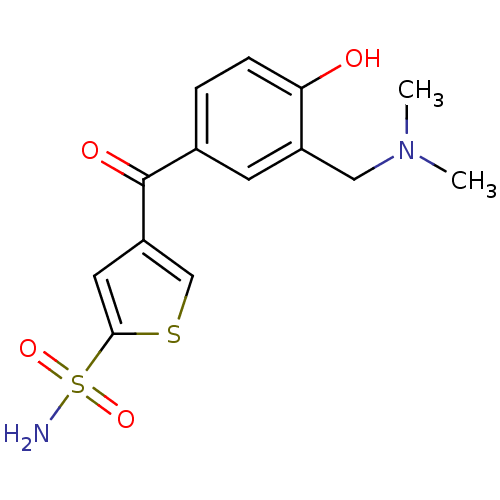 (4-(3-Dimethylaminomethyl-4-hydroxy-benzoyl)-thioph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Compound was tested in vitro for binding affinity against human carbonic anhydrase II; (ki*10e-9) | J Med Chem 35: 3822-31 (1992) BindingDB Entry DOI: 10.7270/Q2ZG6SV4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||