

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50451624 (CHEMBL331897) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50451624 (CHEMBL331897) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity towards C-C chemokine receptor type 1 | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50099482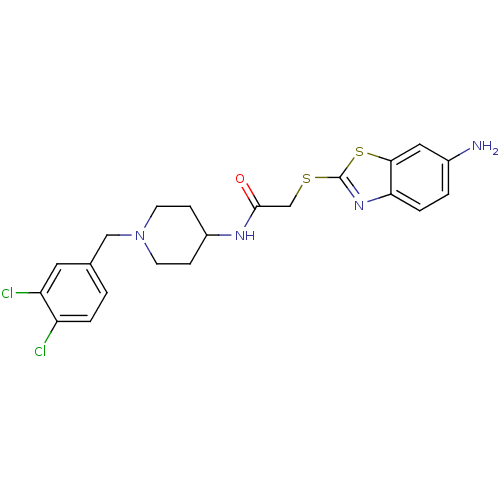 (2-(6-Amino-benzothiazol-2-ylsulfanyl)-N-[1-(3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133793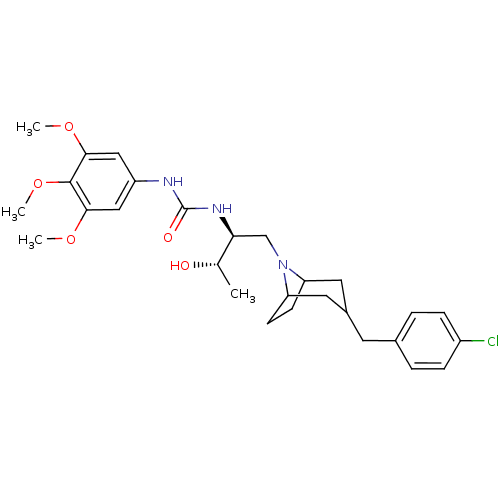 (1-{(S)-1-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133792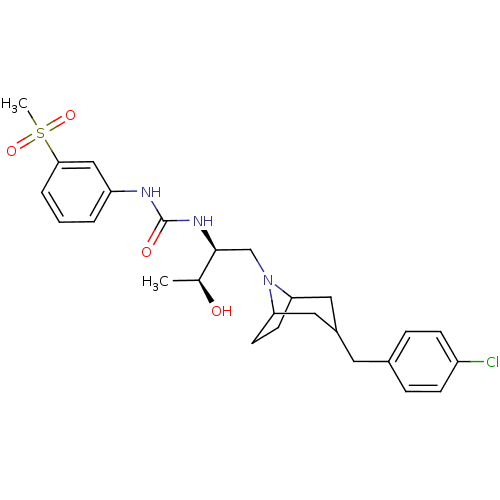 (1-{(S)-1-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133796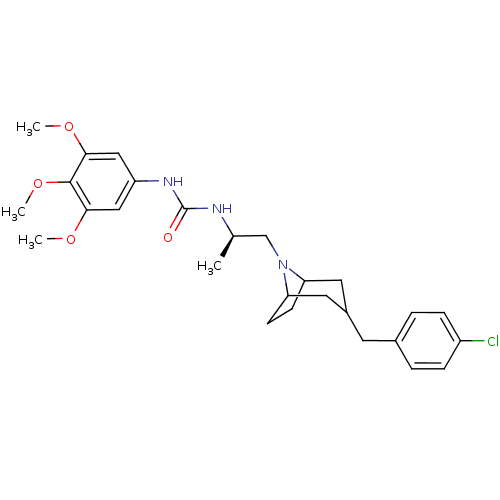 (1-{(R)-2-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50100021 ((S)-2-[(Naphthalene-1-carbonyl)-amino]-3-(4-nitro-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133793 (1-{(S)-1-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133796 (1-{(R)-2-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133789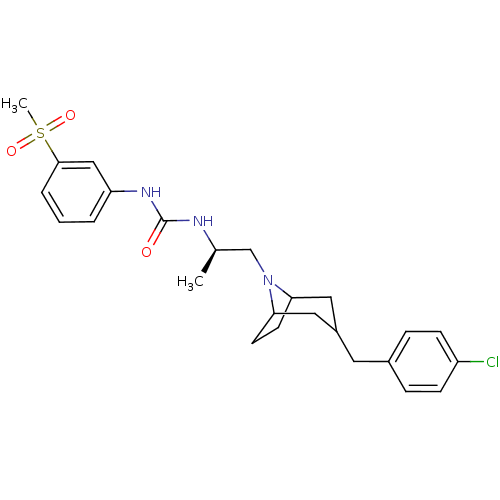 (1-{(R)-2-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit C-C chemokine receptor type 3 receptor | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133792 (1-{(S)-1-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133789 (1-{(R)-2-[3-(4-Chloro-benzyl)-8-aza-bicyclo[3.2.1]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133800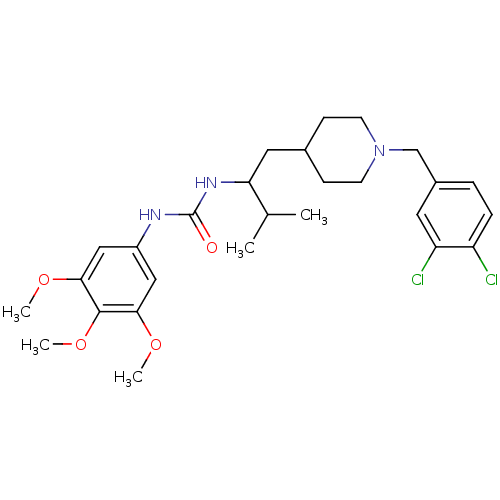 (1-{1-[1-(3,4-Dichloro-benzyl)-piperidin-4-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133795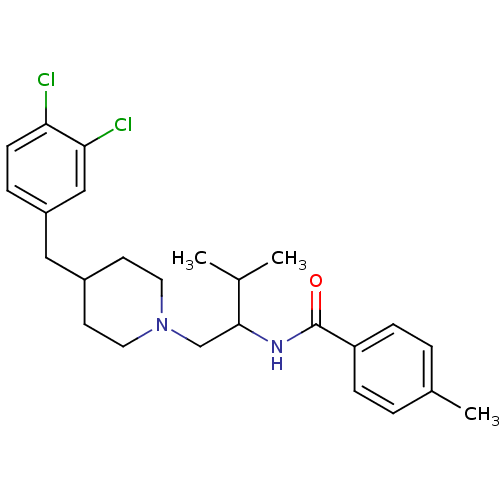 (CHEMBL123943 | N-{1-[4-(3,4-Dichloro-benzyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133798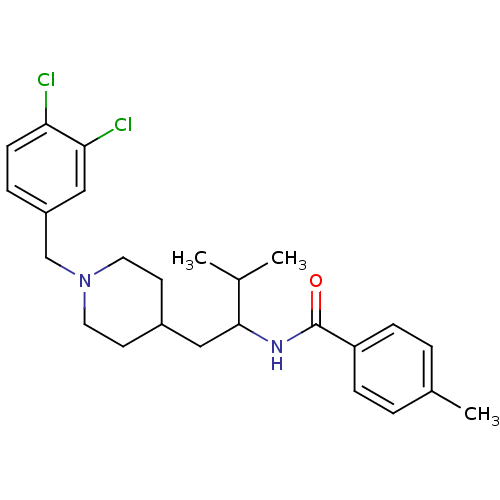 (CHEMBL331702 | N-{1-[1-(3,4-Dichloro-benzyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133790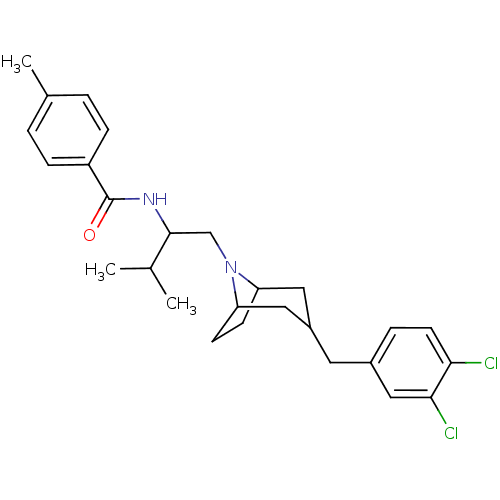 (CHEMBL123308 | N-{1-[3-(3,4-Dichloro-benzyl)-8-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133788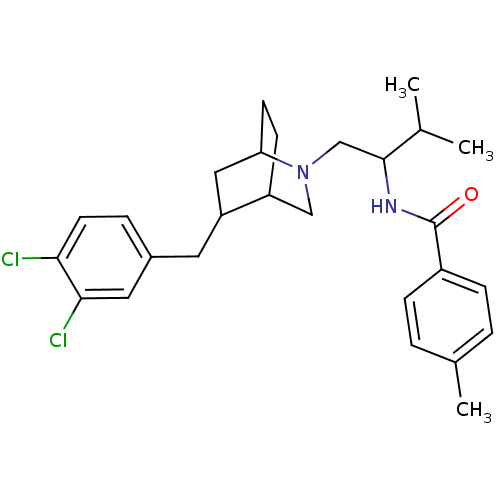 (CHEMBL331672 | N-{1-[5-(3,4-Dichloro-benzyl)-2-aza...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133791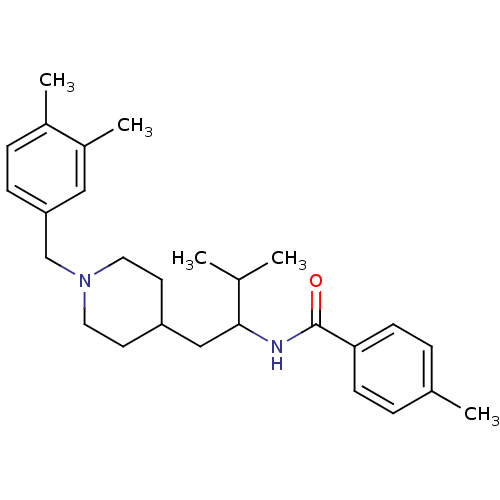 (CHEMBL120957 | N-{1-[1-(3,4-Dimethyl-benzyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133799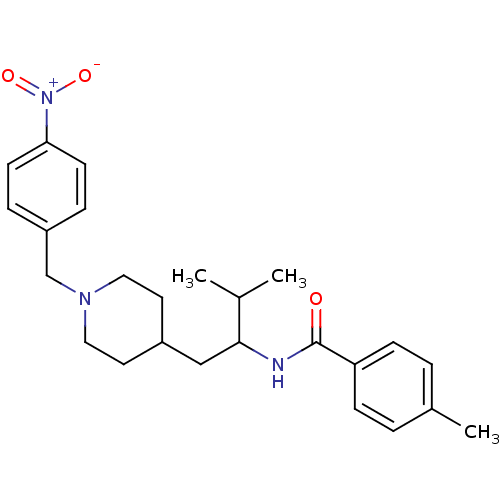 (4-Methyl-N-{2-methyl-1-[1-(4-nitro-benzyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133797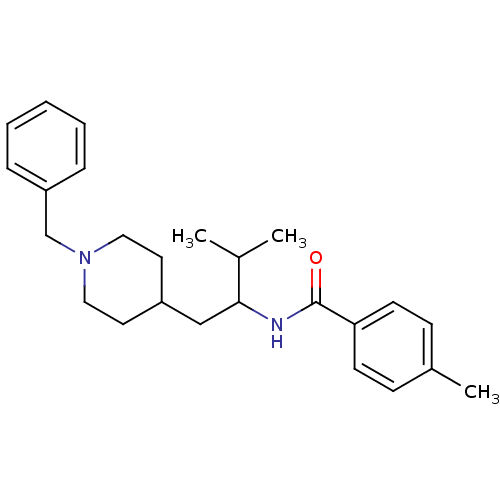 (CHEMBL121743 | N-[1-(1-Benzyl-piperidin-4-ylmethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50133794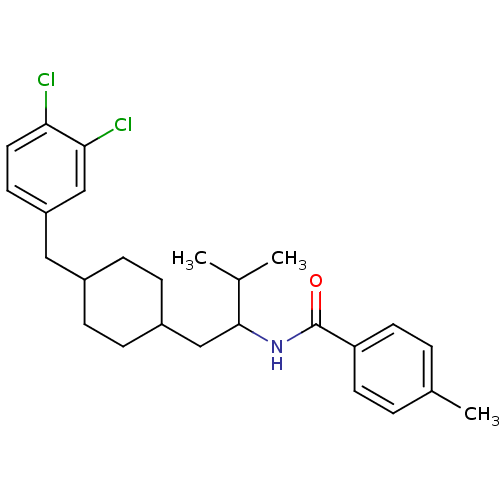 (CHEMBL121588 | N-{1-[4-(3,4-Dichloro-benzyl)-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto Curated by ChEMBL | Assay Description Binding affinity was determined towards C-C chemokine receptor type 3 using [125I]-labeled eotaxin as radioligand | Bioorg Med Chem Lett 13: 3597-600 (2003) BindingDB Entry DOI: 10.7270/Q228085J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||