

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM13246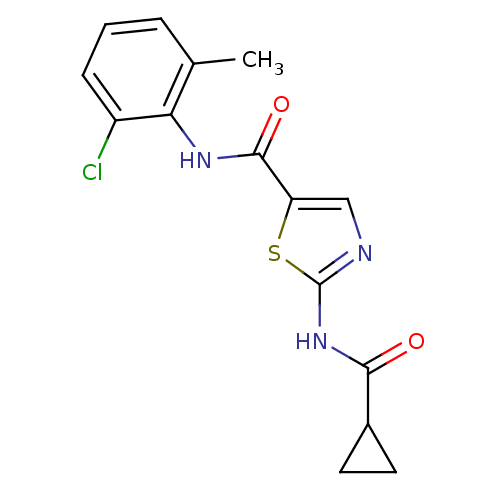 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Src kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50135404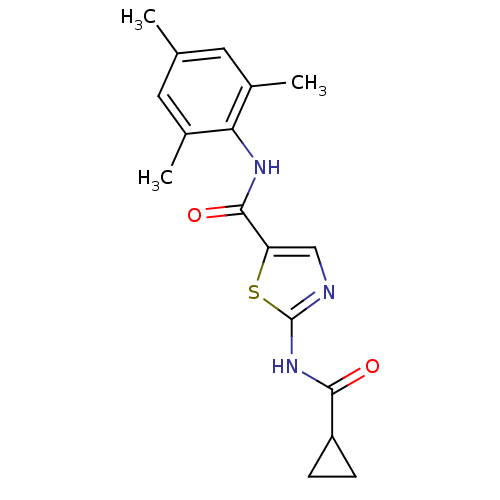 (2-(Cyclopropanecarbonyl-amino)-thiazole-5-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13242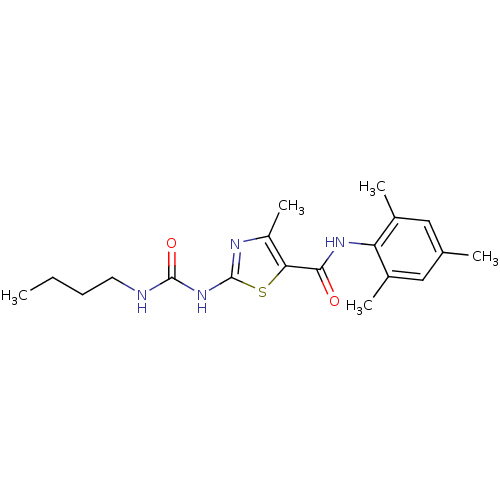 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Lck kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Jak3 kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13242 (2-[(butylcarbamoyl)amino]-4-methyl-N-(2,4,6-trimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Fyn (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Fyn kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13240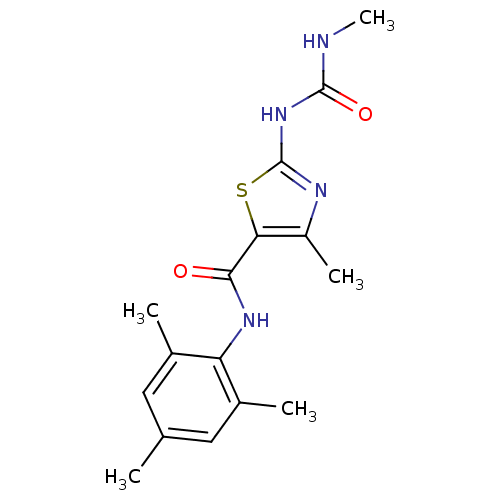 (2-[[(Methylamino)-carbonyl]amino]-4-methyl-N-(2,4,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13240 (2-[[(Methylamino)-carbonyl]amino]-4-methyl-N-(2,4,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase HCK (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of Hck kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13224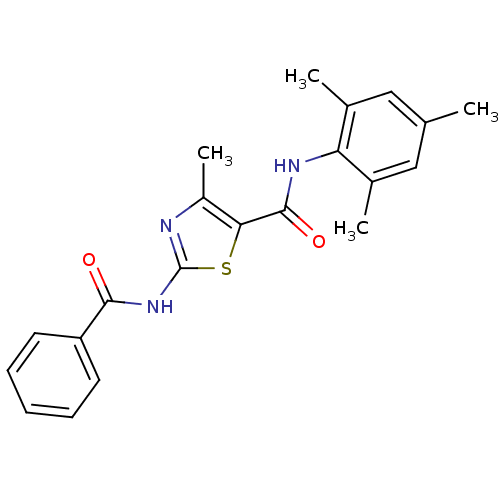 (2-N-benzene-4-methyl-5-N-(2,4,6-trimethylphenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13232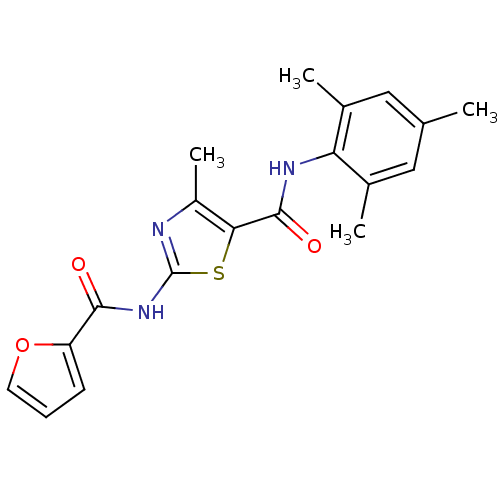 (2-(2-furoylamino)-N-mesityl-4-methyl-1,3-thiazole-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13245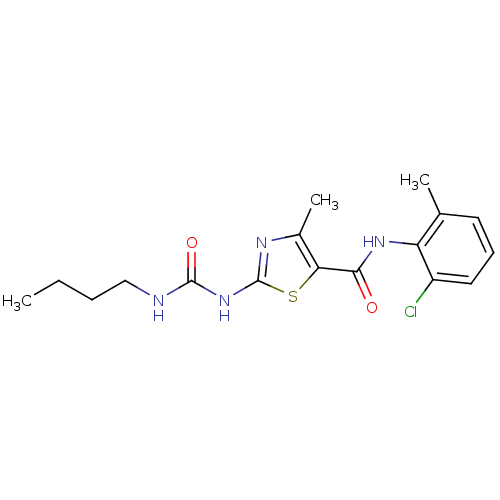 (2-[(butylcarbamoyl)amino]-N-(2-chloro-6-methylphen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13222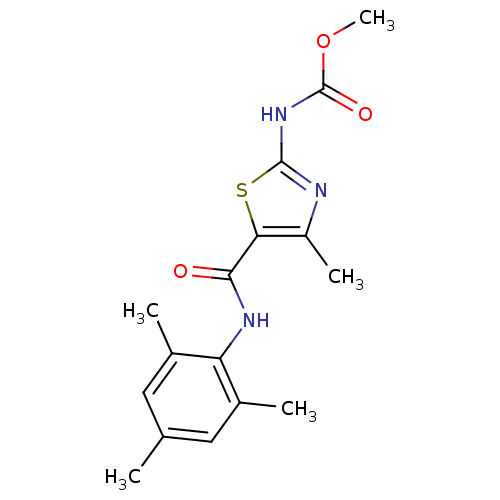 (2-[[(Methoxy)carbonyl]-amino]-4-methyl-N-(2,4,6-tr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13237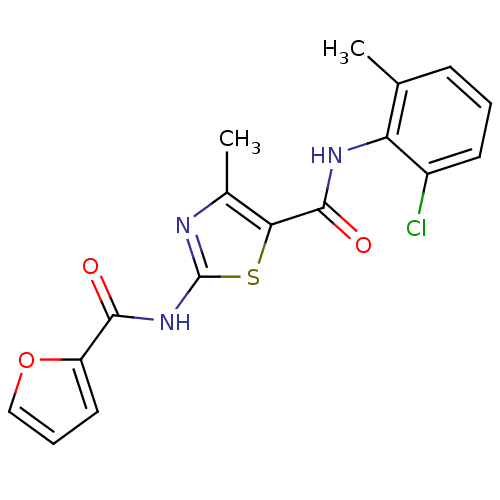 (5-N-(2-chloro-6-methylphenyl)-2-N-(furan-2-)-4-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13222 (2-[[(Methoxy)carbonyl]-amino]-4-methyl-N-(2,4,6-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13224 (2-N-benzene-4-methyl-5-N-(2,4,6-trimethylphenyl)-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of CD3/CD28 T-cell proliferation assay in PBL (peripheral blood lymphocytes) | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13250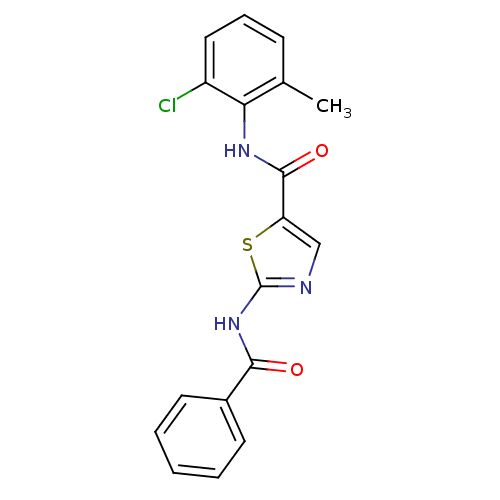 (2-N-benzene-5-N-(2-chloro-6-methylphenyl)-1,3-thia...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13220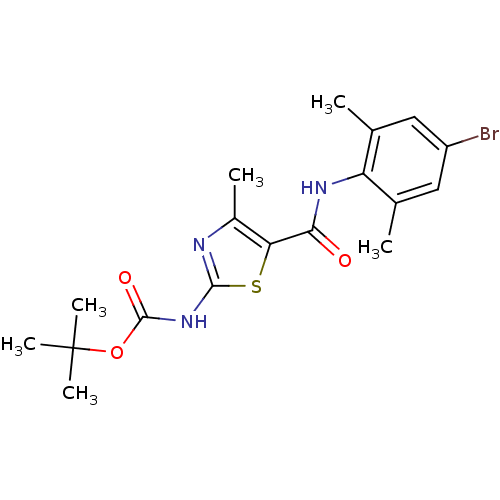 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(4-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13239 (2-[[(Cyclopropyl)carbonyl]-amino]-4-methyl-N-(2-ch...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13217 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13220 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13217 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13253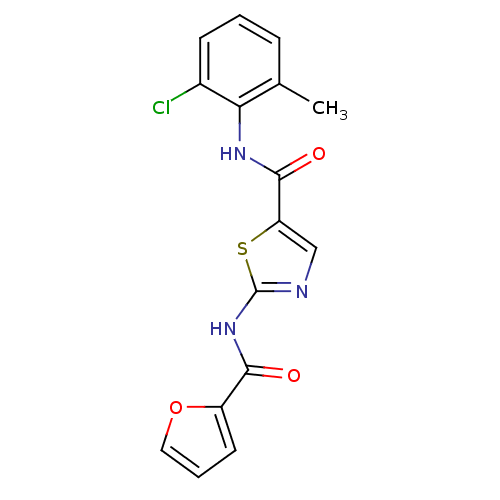 (5-N-(2-chloro-6-methylphenyl)-2-N-(furan-2-)-1,3-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13219 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13238 (2-N-benzene-5-N-(2-chloro-6-methylphenyl)-4-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13215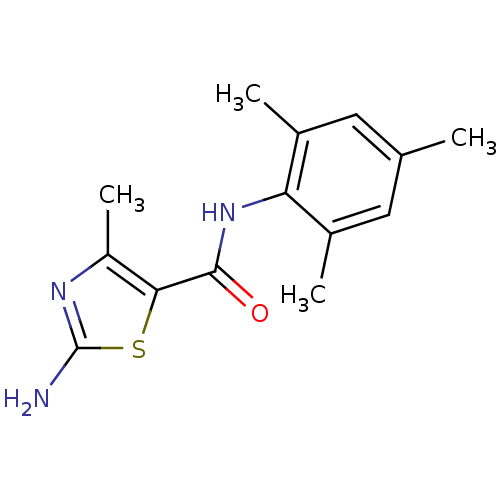 (2-Aminothiazole 1 | 2-amino-4-methyl-N-(2,4,6-trim...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13215 (2-Aminothiazole 1 | 2-amino-4-methyl-N-(2,4,6-trim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13219 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13218 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of mouse p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound against HER1 kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13221 (BMS-354825 tert-Butoxycarbamate Analog 5e | CHEMBL...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13228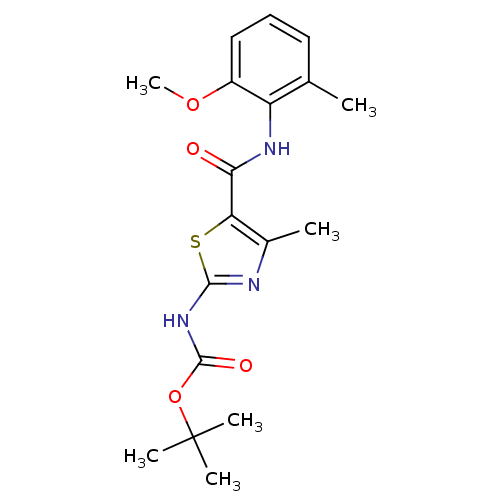 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK2 kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of FAK kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 1/2/3/4 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of FGF receptor | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of KDR kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 11/12/13/14 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM13246 (5-N-(2-chloro-6-methylphenyl)-2-N-cyclopropane-1,3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of HER2 kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM13218 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of human p56 Lck tyrosine kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50135407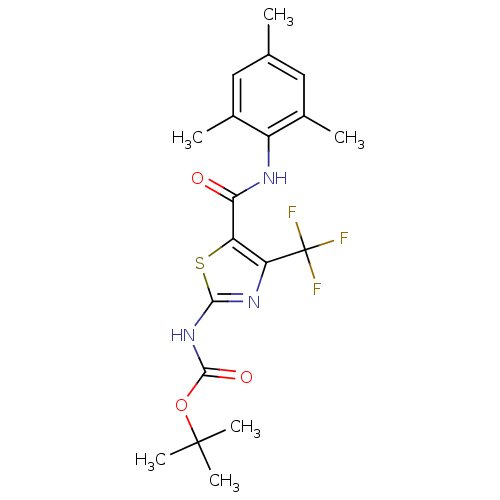 (CHEMBL128581 | [4-Trifluoromethyl-5-(2,4,6-trimeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50135402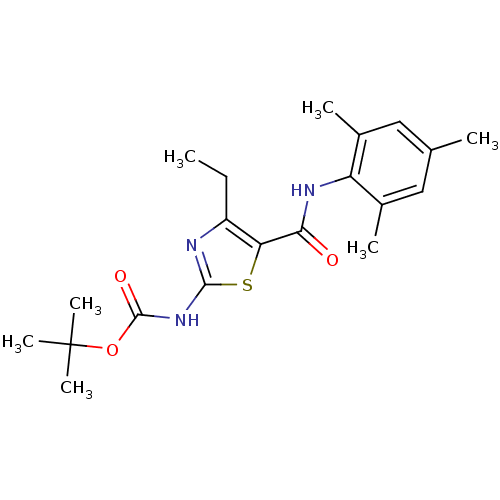 (CHEMBL130971 | [4-Ethyl-5-(2,4,6-trimethyl-phenylc...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50135397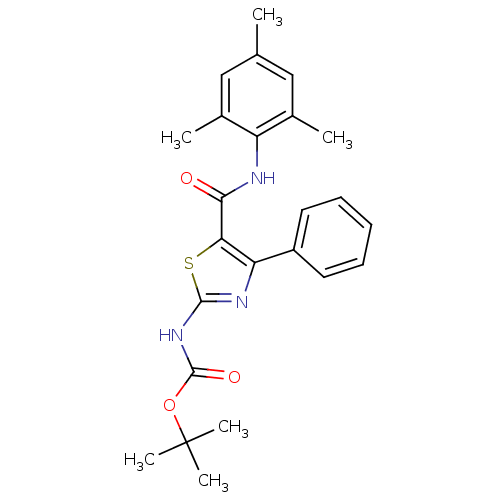 (CHEMBL340773 | [4-Phenyl-5-(2,4,6-trimethyl-phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50135403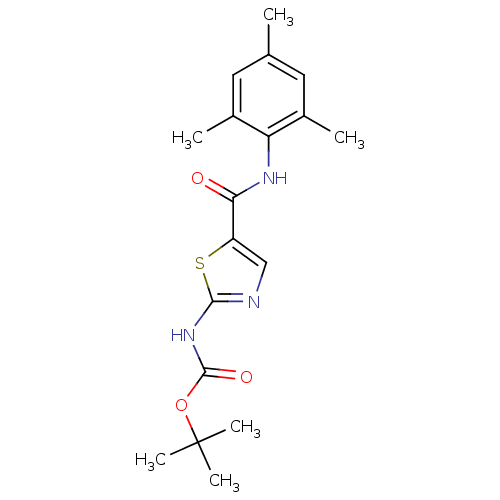 (CHEMBL130320 | [5-(2,4,6-Trimethyl-phenylcarbamoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13227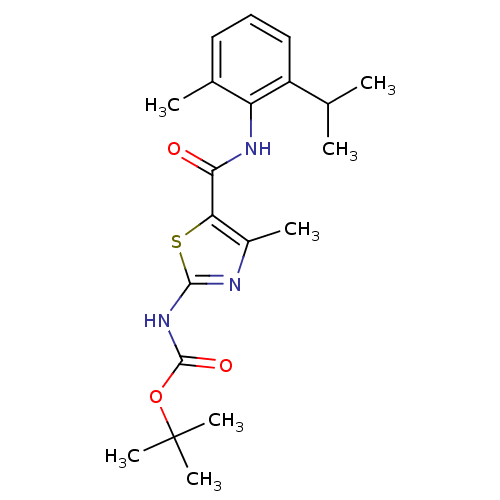 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13226 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-(2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135401 (CHEMBL130943 | [5-(3-Methoxy-5-trifluoromethyl-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50135406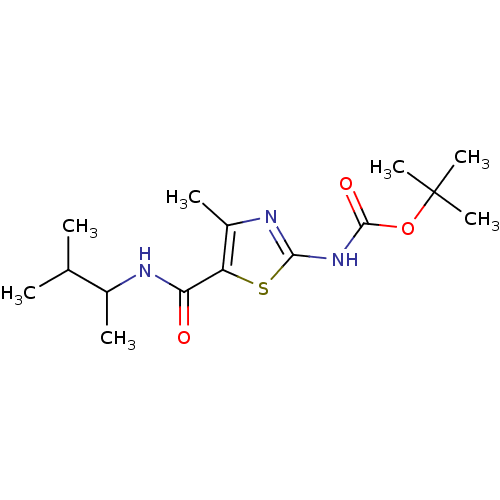 (CHEMBL131167 | [5-(1,2-Dimethyl-propylcarbamoyl)-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound human Lck(hLck) kinase | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135409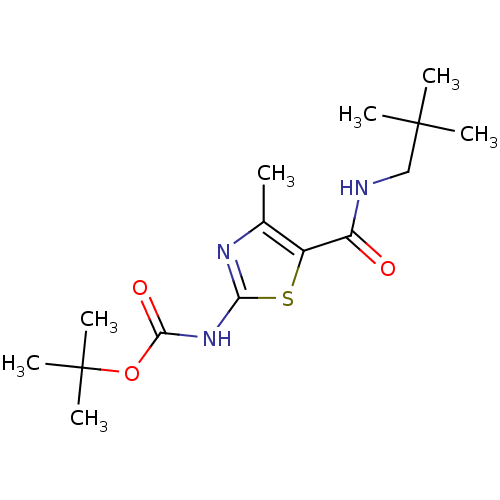 (CHEMBL128795 | [5-(2,2-Dimethyl-propylcarbamoyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135398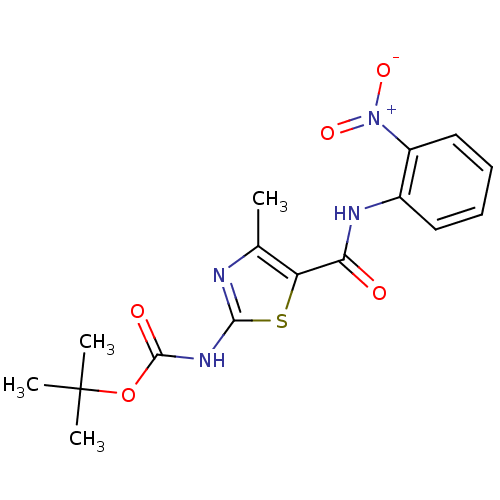 (CHEMBL423516 | [4-Methyl-5-(2-nitro-phenylcarbamoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135405 (CHEMBL334341 | [5-(2,6-Diisopropyl-phenylcarbamoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135399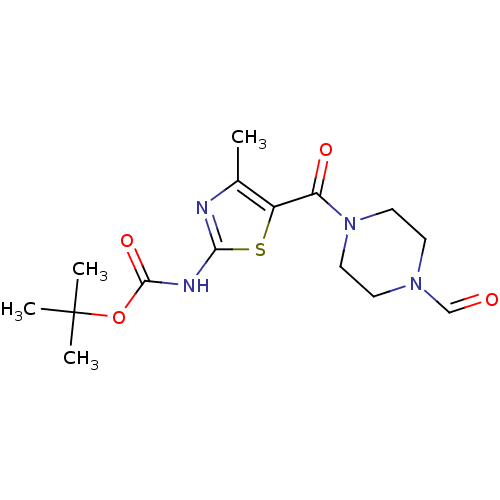 (CHEMBL130075 | [5-(4-Formyl-piperazine-1-carbonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135400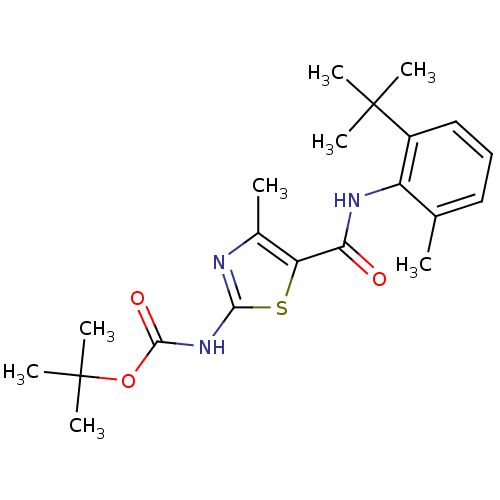 (CHEMBL338488 | [5-(2-tert-Butyl-6-methyl-phenylcar...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135396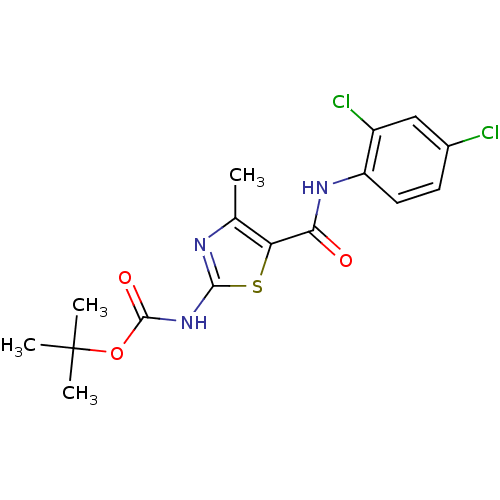 (CHEMBL336544 | [5-(2,4-Dichloro-phenylcarbamoyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135408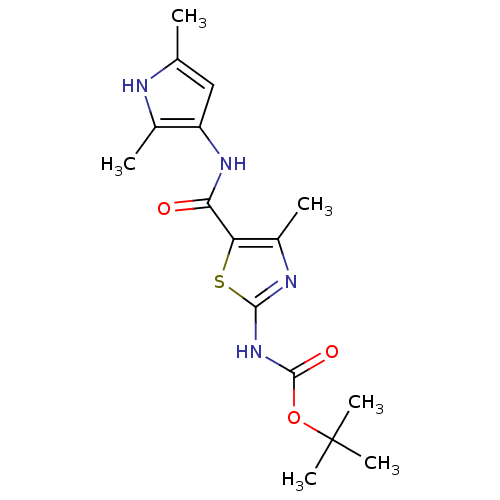 (CHEMBL128455 | [5-(2,5-Dimethyl-1H-pyrrol-3-ylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135395 (CHEMBL341026 | [5-(Cyclohexylmethyl-carbamoyl)-4-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM50135406 (CHEMBL131167 | [5-(1,2-Dimethyl-propylcarbamoyl)-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase LCK (Mus musculus) | BDBM13225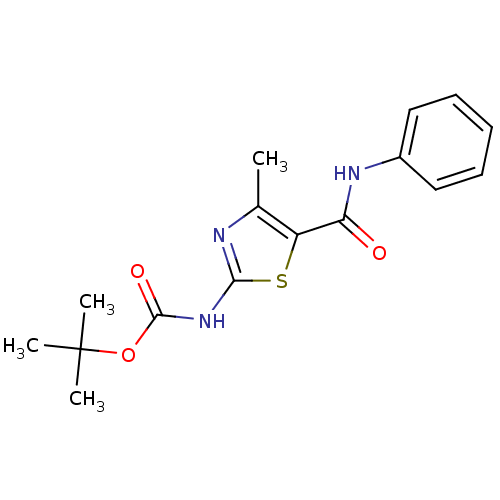 (2-[[(tert-Butyloxy)carbonyl]-amino]-4-methyl-N-phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of murine Lck kinase. | Bioorg Med Chem Lett 13: 4007-10 (2003) BindingDB Entry DOI: 10.7270/Q2CC103J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||