 Found 50 hits of Enzyme Inhibition Constant Data
Found 50 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135558
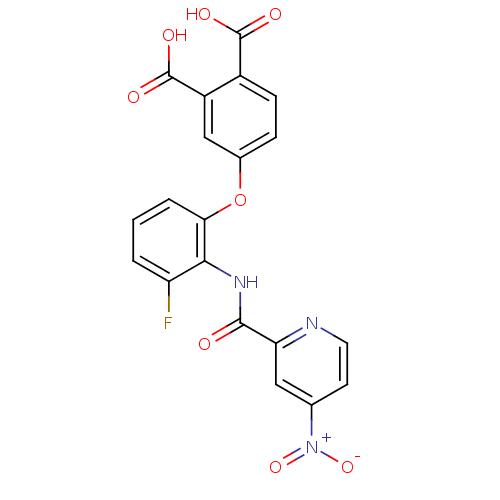
(4-{3-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-14-2-1-3-16(32-11-4-5-12(19(26)27)13(9-11)20(28)29)17(14)23-18(25)15-8-10(24(30)31)6-7-22-15/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135554

(4-{3-[(4-Nitro-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C24H15N3O8/c28-22(20-11-15(27(33)34)7-8-25-20)26-19-9-13-3-1-2-4-14(13)10-21(19)35-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135553
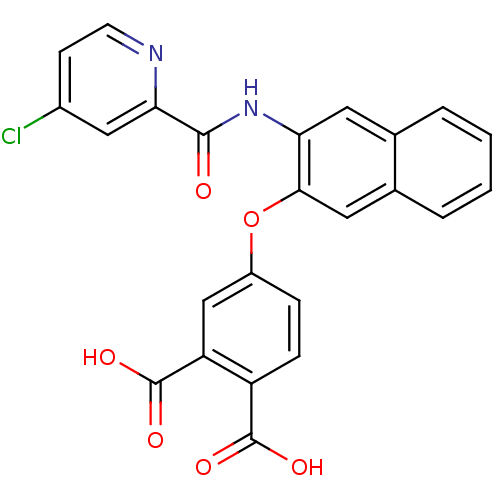
(4-{3-[(4-Chloro-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C24H15ClN2O6/c25-15-7-8-26-20(11-15)22(28)27-19-9-13-3-1-2-4-14(13)10-21(19)33-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135565
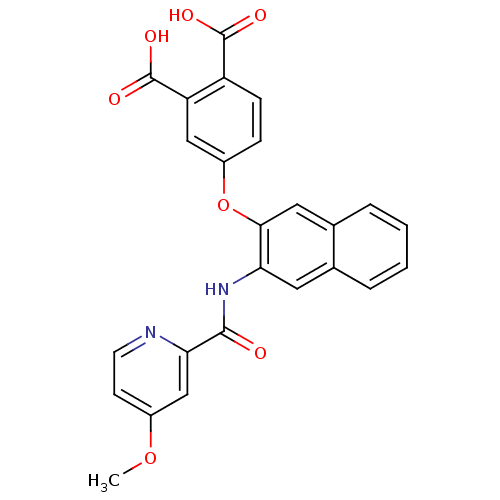
(4-{3-[(4-Methoxy-pyridine-2-carbonyl)-amino]-napht...)Show SMILES COc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O7/c1-33-16-8-9-26-21(13-16)23(28)27-20-10-14-4-2-3-5-15(14)11-22(20)34-17-6-7-18(24(29)30)19(12-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135554

(4-{3-[(4-Nitro-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C24H15N3O8/c28-22(20-11-15(27(33)34)7-8-25-20)26-19-9-13-3-1-2-4-14(13)10-21(19)35-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135562
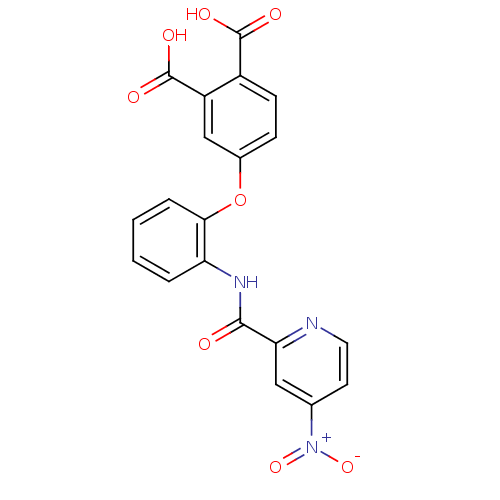
(4-{2-[(4-Nitro-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H13N3O8/c24-18(16-9-11(23(29)30)7-8-21-16)22-15-3-1-2-4-17(15)31-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135557
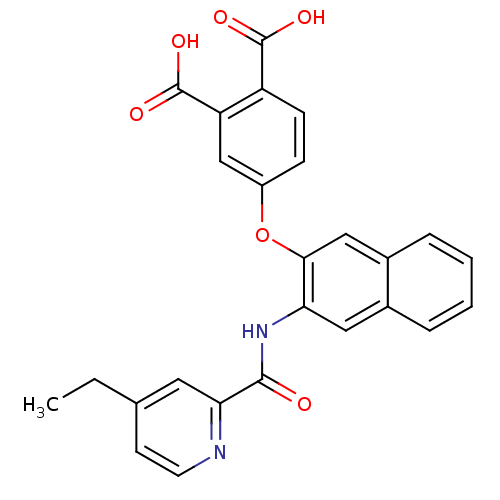
(4-{3-[(4-Ethyl-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES CCc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C26H20N2O6/c1-2-15-9-10-27-22(11-15)24(29)28-21-12-16-5-3-4-6-17(16)13-23(21)34-18-7-8-19(25(30)31)20(14-18)26(32)33/h3-14H,2H2,1H3,(H,28,29)(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135558

(4-{3-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-14-2-1-3-16(32-11-4-5-12(19(26)27)13(9-11)20(28)29)17(14)23-18(25)15-8-10(24(30)31)6-7-22-15/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50135550
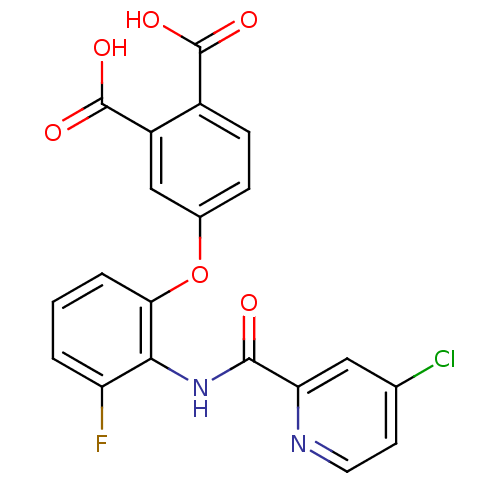
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135550

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135550

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135560
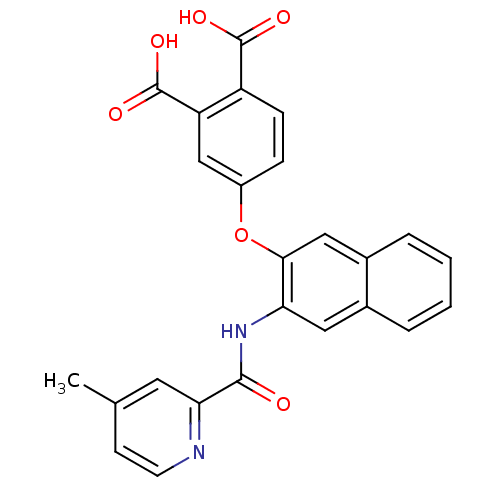
(4-{3-[(4-Methyl-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES Cc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O6/c1-14-8-9-26-21(10-14)23(28)27-20-11-15-4-2-3-5-16(15)12-22(20)33-17-6-7-18(24(29)30)19(13-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135561
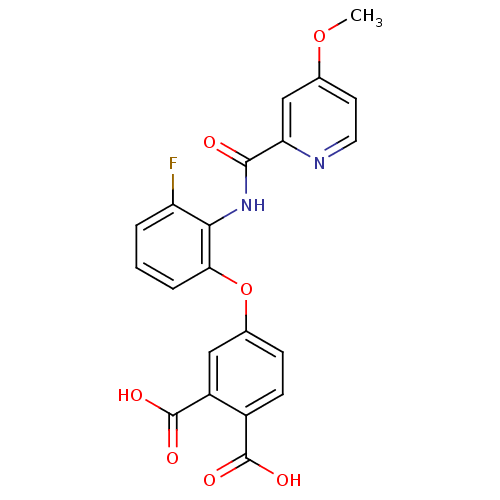
(4-{3-Fluoro-2-[(4-methoxy-pyridine-2-carbonyl)-ami...)Show SMILES COc1ccnc(c1)C(=O)Nc1c(F)cccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H15FN2O7/c1-30-11-7-8-23-16(10-11)19(25)24-18-15(22)3-2-4-17(18)31-12-5-6-13(20(26)27)14(9-12)21(28)29/h2-10H,1H3,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135553

(4-{3-[(4-Chloro-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C24H15ClN2O6/c25-15-7-8-26-20(11-15)22(28)27-19-9-13-3-1-2-4-14(13)10-21(19)33-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135565

(4-{3-[(4-Methoxy-pyridine-2-carbonyl)-amino]-napht...)Show SMILES COc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O7/c1-33-16-8-9-26-21(13-16)23(28)27-20-10-14-4-2-3-5-15(14)11-22(20)34-17-6-7-18(24(29)30)19(12-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135567
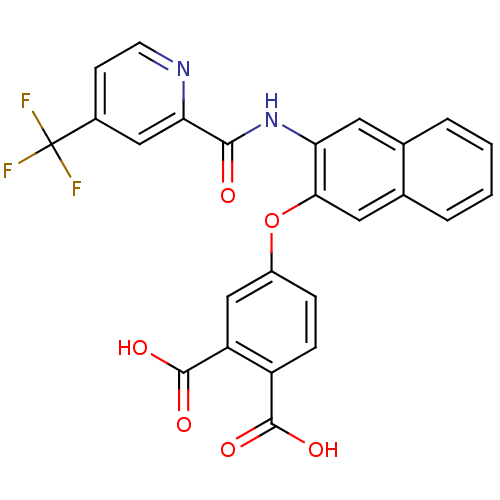
(4-{3-[(4-Trifluoromethyl-pyridine-2-carbonyl)-amin...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)C(F)(F)F)cc1C(O)=O Show InChI InChI=1S/C25H15F3N2O6/c26-25(27,28)15-7-8-29-20(11-15)22(31)30-19-9-13-3-1-2-4-14(13)10-21(19)36-16-5-6-17(23(32)33)18(12-16)24(34)35/h1-12H,(H,30,31)(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135559
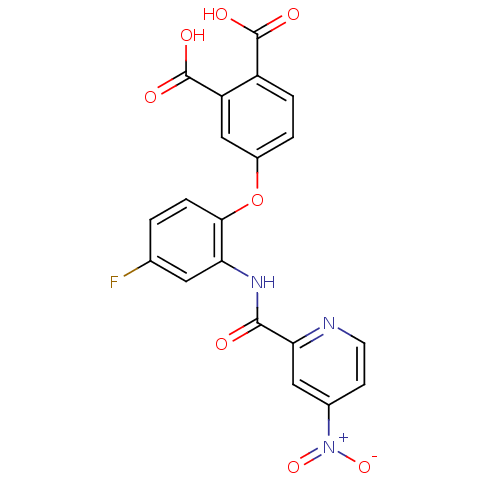
(4-{4-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2ccc(F)cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-10-1-4-17(32-12-2-3-13(19(26)27)14(9-12)20(28)29)15(7-10)23-18(25)16-8-11(24(30)31)5-6-22-16/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135552
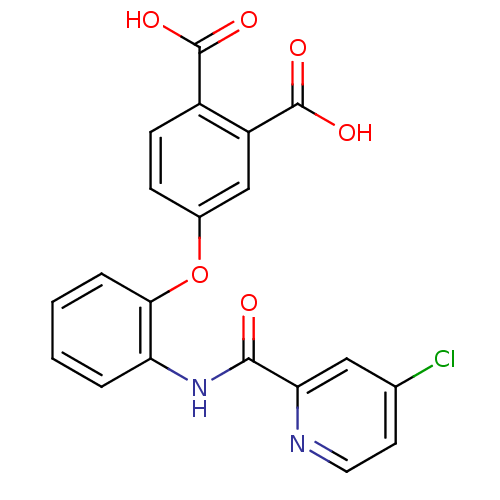
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-phenox...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13ClN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135563
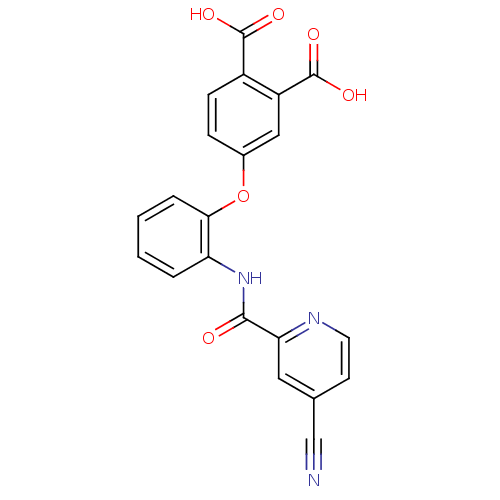
(4-{2-[(4-Cyano-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)C#N)cc1C(O)=O Show InChI InChI=1S/C21H13N3O6/c22-11-12-7-8-23-17(9-12)19(25)24-16-3-1-2-4-18(16)30-13-5-6-14(20(26)27)15(10-13)21(28)29/h1-10H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50135552

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-phenox...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13ClN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135563

(4-{2-[(4-Cyano-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)C#N)cc1C(O)=O Show InChI InChI=1S/C21H13N3O6/c22-11-12-7-8-23-17(9-12)19(25)24-16-3-1-2-4-18(16)30-13-5-6-14(20(26)27)15(10-13)21(28)29/h1-10H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135552

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-phenox...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13ClN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135556
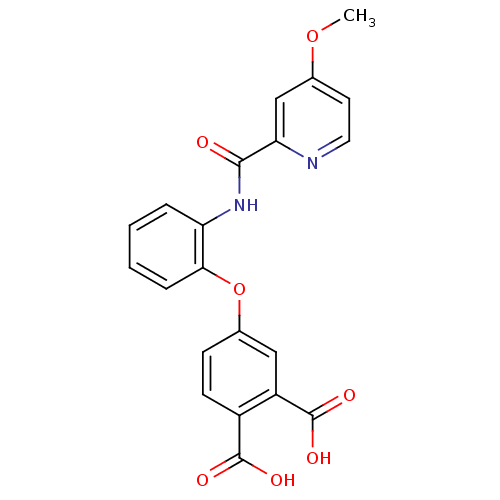
(4-{2-[(4-Methoxy-pyridine-2-carbonyl)-amino]-pheno...)Show SMILES COc1ccnc(c1)C(=O)Nc1ccccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H16N2O7/c1-29-12-8-9-22-17(11-12)19(24)23-16-4-2-3-5-18(16)30-13-6-7-14(20(25)26)15(10-13)21(27)28/h2-11H,1H3,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50135563

(4-{2-[(4-Cyano-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)C#N)cc1C(O)=O Show InChI InChI=1S/C21H13N3O6/c22-11-12-7-8-23-17(9-12)19(25)24-16-3-1-2-4-18(16)30-13-5-6-14(20(26)27)15(10-13)21(28)29/h1-10H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135556

(4-{2-[(4-Methoxy-pyridine-2-carbonyl)-amino]-pheno...)Show SMILES COc1ccnc(c1)C(=O)Nc1ccccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H16N2O7/c1-29-12-8-9-22-17(11-12)19(24)23-16-4-2-3-5-18(16)30-13-6-7-14(20(25)26)15(10-13)21(27)28/h2-11H,1H3,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135568
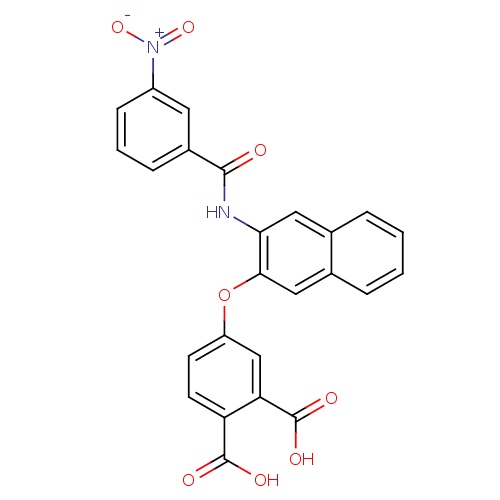
(4-[3-(3-Nitro-benzoylamino)-naphthalen-2-yloxy]-ph...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C25H16N2O8/c28-23(16-6-3-7-17(10-16)27(33)34)26-21-11-14-4-1-2-5-15(14)12-22(21)35-18-8-9-19(24(29)30)20(13-18)25(31)32/h1-13H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50135556

(4-{2-[(4-Methoxy-pyridine-2-carbonyl)-amino]-pheno...)Show SMILES COc1ccnc(c1)C(=O)Nc1ccccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H16N2O7/c1-29-12-8-9-22-17(11-12)19(24)23-16-4-2-3-5-18(16)30-13-6-7-14(20(25)26)15(10-13)21(27)28/h2-11H,1H3,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135562

(4-{2-[(4-Nitro-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H13N3O8/c24-18(16-9-11(23(29)30)7-8-21-16)22-15-3-1-2-4-17(15)31-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135557

(4-{3-[(4-Ethyl-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES CCc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C26H20N2O6/c1-2-15-9-10-27-22(11-15)24(29)28-21-12-16-5-3-4-6-17(16)13-23(21)34-18-7-8-19(25(30)31)20(14-18)26(32)33/h3-14H,2H2,1H3,(H,28,29)(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135551
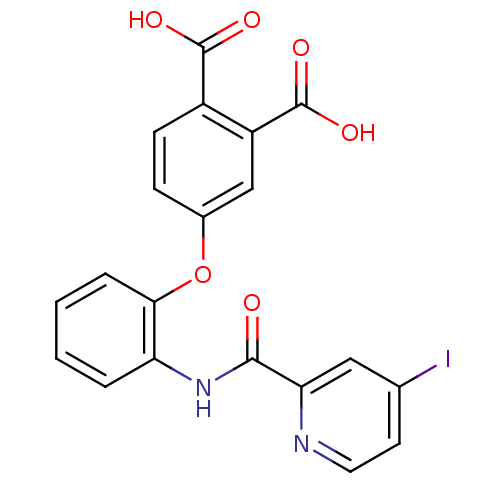
(4-{2-[(4-Iodo-pyridine-2-carbonyl)-amino]-phenoxy}...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(I)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13IN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135560

(4-{3-[(4-Methyl-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES Cc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O6/c1-14-8-9-26-21(10-14)23(28)27-20-11-15-4-2-3-5-16(15)12-22(20)33-17-6-7-18(24(29)30)19(13-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135550

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135555
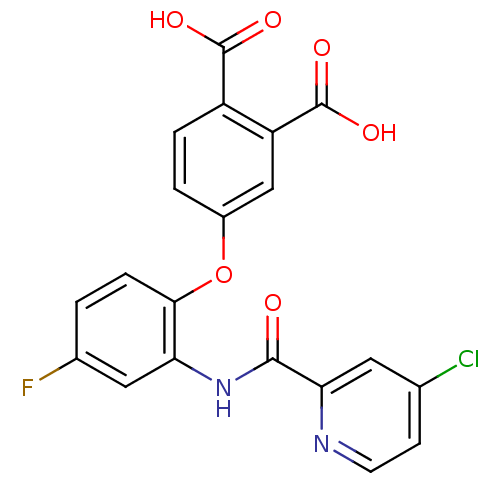
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-4-fluo...)Show SMILES OC(=O)c1ccc(Oc2ccc(F)cc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-5-6-23-16(7-10)18(25)24-15-8-11(22)1-4-17(15)30-12-2-3-13(19(26)27)14(9-12)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135567

(4-{3-[(4-Trifluoromethyl-pyridine-2-carbonyl)-amin...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)C(F)(F)F)cc1C(O)=O Show InChI InChI=1S/C25H15F3N2O6/c26-25(27,28)15-7-8-29-20(11-15)22(31)30-19-9-13-3-1-2-4-14(13)10-21(19)36-16-5-6-17(23(32)33)18(12-16)24(34)35/h1-12H,(H,30,31)(H,32,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135559

(4-{4-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2ccc(F)cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-10-1-4-17(32-12-2-3-13(19(26)27)14(9-12)20(28)29)15(7-10)23-18(25)16-8-11(24(30)31)5-6-22-16/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135561

(4-{3-Fluoro-2-[(4-methoxy-pyridine-2-carbonyl)-ami...)Show SMILES COc1ccnc(c1)C(=O)Nc1c(F)cccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H15FN2O7/c1-30-11-7-8-23-16(10-11)19(25)24-18-15(22)3-2-4-17(18)31-12-5-6-13(20(26)27)14(9-12)21(28)29/h2-10H,1H3,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135568

(4-[3-(3-Nitro-benzoylamino)-naphthalen-2-yloxy]-ph...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C25H16N2O8/c28-23(16-6-3-7-17(10-16)27(33)34)26-21-11-14-4-1-2-5-15(14)12-22(21)35-18-8-9-19(24(29)30)20(13-18)25(31)32/h1-13H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135563

(4-{2-[(4-Cyano-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)C#N)cc1C(O)=O Show InChI InChI=1S/C21H13N3O6/c22-11-12-7-8-23-17(9-12)19(25)24-16-3-1-2-4-18(16)30-13-5-6-14(20(26)27)15(10-13)21(28)29/h1-10H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135564
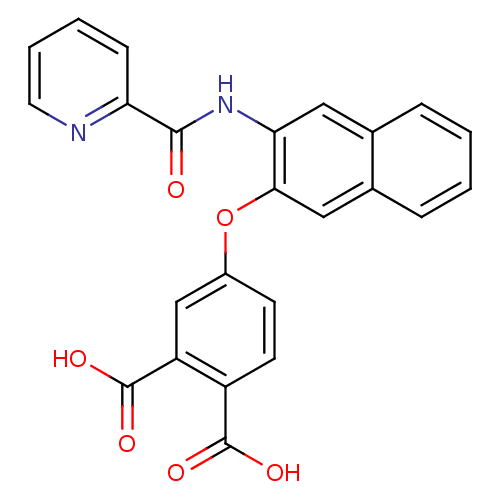
(4-{3-[(Pyridine-2-carbonyl)-amino]-naphthalen-2-yl...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2ccccn2)cc1C(O)=O Show InChI InChI=1S/C24H16N2O6/c27-22(19-7-3-4-10-25-19)26-20-11-14-5-1-2-6-15(14)12-21(20)32-16-8-9-17(23(28)29)18(13-16)24(30)31/h1-13H,(H,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135552

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-phenox...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13ClN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 181 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135556

(4-{2-[(4-Methoxy-pyridine-2-carbonyl)-amino]-pheno...)Show SMILES COc1ccnc(c1)C(=O)Nc1ccccc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C21H16N2O7/c1-29-12-8-9-22-17(11-12)19(24)23-16-4-2-3-5-18(16)30-13-6-7-14(20(25)26)15(10-13)21(27)28/h2-11H,1H3,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135549
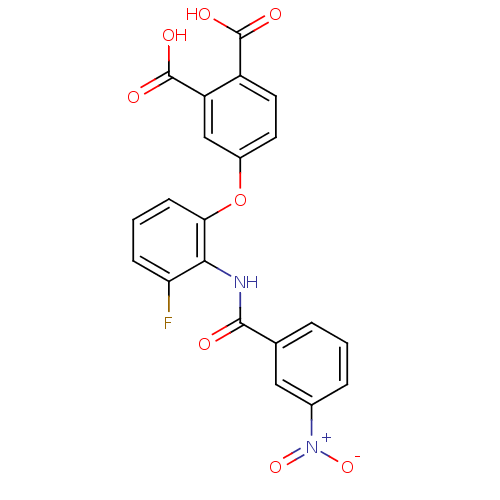
(4-[3-Fluoro-2-(3-nitro-benzoylamino)-phenoxy]-phth...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C21H13FN2O8/c22-16-5-2-6-17(32-13-7-8-14(20(26)27)15(10-13)21(28)29)18(16)23-19(25)11-3-1-4-12(9-11)24(30)31/h1-10H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 207 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135549

(4-[3-Fluoro-2-(3-nitro-benzoylamino)-phenoxy]-phth...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C21H13FN2O8/c22-16-5-2-6-17(32-13-7-8-14(20(26)27)15(10-13)21(28)29)18(16)23-19(25)11-3-1-4-12(9-11)24(30)31/h1-10H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50135549

(4-[3-Fluoro-2-(3-nitro-benzoylamino)-phenoxy]-phth...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C21H13FN2O8/c22-16-5-2-6-17(32-13-7-8-14(20(26)27)15(10-13)21(28)29)18(16)23-19(25)11-3-1-4-12(9-11)24(30)31/h1-10H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 302 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135551

(4-{2-[(4-Iodo-pyridine-2-carbonyl)-amino]-phenoxy}...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(I)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H13IN2O6/c21-11-7-8-22-16(9-11)18(24)23-15-3-1-2-4-17(15)29-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,23,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 468 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135555

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-4-fluo...)Show SMILES OC(=O)c1ccc(Oc2ccc(F)cc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-5-6-23-16(7-10)18(25)24-15-8-11(22)1-4-17(15)30-12-2-3-13(19(26)27)14(9-12)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 647 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135564

(4-{3-[(Pyridine-2-carbonyl)-amino]-naphthalen-2-yl...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2ccccn2)cc1C(O)=O Show InChI InChI=1S/C24H16N2O6/c27-22(19-7-3-4-10-25-19)26-20-11-14-5-1-2-6-15(14)12-21(20)32-16-8-9-17(23(28)29)18(13-16)24(30)31/h1-13H,(H,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 844 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135549

(4-[3-Fluoro-2-(3-nitro-benzoylamino)-phenoxy]-phth...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cccc(c2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C21H13FN2O8/c22-16-5-2-6-17(32-13-7-8-14(20(26)27)15(10-13)21(28)29)18(16)23-19(25)11-3-1-4-12(9-11)24(30)31/h1-10H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135566
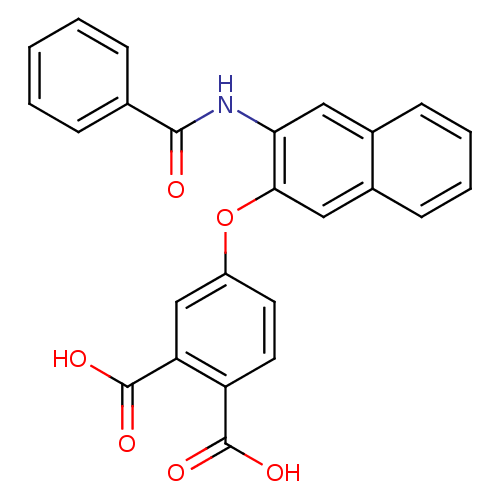
(4-(3-Benzoylamino-naphthalen-2-yloxy)-phthalic aci...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2ccccc2)cc1C(O)=O Show InChI InChI=1S/C25H17NO6/c27-23(15-6-2-1-3-7-15)26-21-12-16-8-4-5-9-17(16)13-22(21)32-18-10-11-19(24(28)29)20(14-18)25(30)31/h1-14H,(H,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135566

(4-(3-Benzoylamino-naphthalen-2-yloxy)-phthalic aci...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2ccccc2)cc1C(O)=O Show InChI InChI=1S/C25H17NO6/c27-23(15-6-2-1-3-7-15)26-21-12-16-8-4-5-9-17(16)13-22(21)32-18-10-11-19(24(28)29)20(14-18)25(30)31/h1-14H,(H,26,27)(H,28,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data