

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9409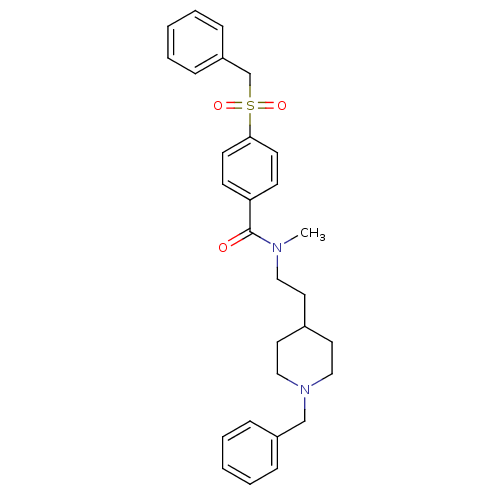 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004016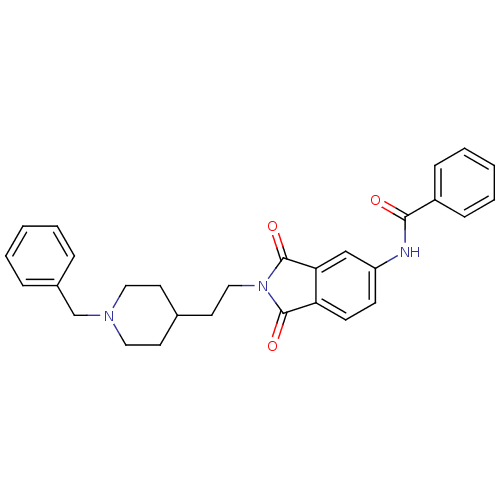 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004007 (2-[2-(1-Benzyl-piperidin-4-yl)-ethyl]-1,3-dioxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004001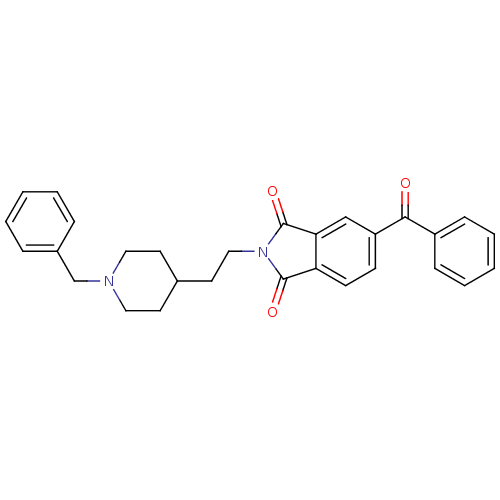 (5-Benzoyl-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-is...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004035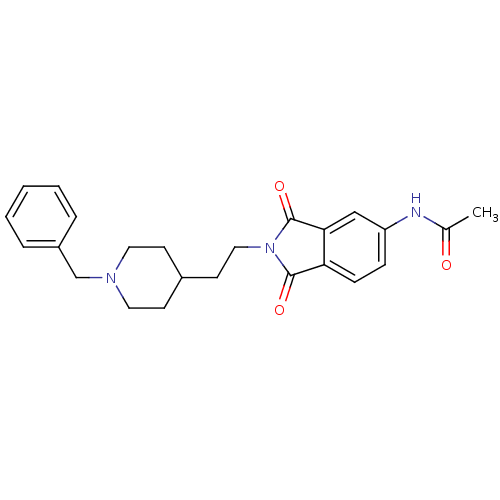 (CHEMBL433678 | N-(2-(2-(1-benzylpiperidin-4-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004018 (3-(2-(1-benzylpiperidin-4-yl)ethyl)quinazoline-2,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003999 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-7-chloroquinaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003996 (CHEMBL126354 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004011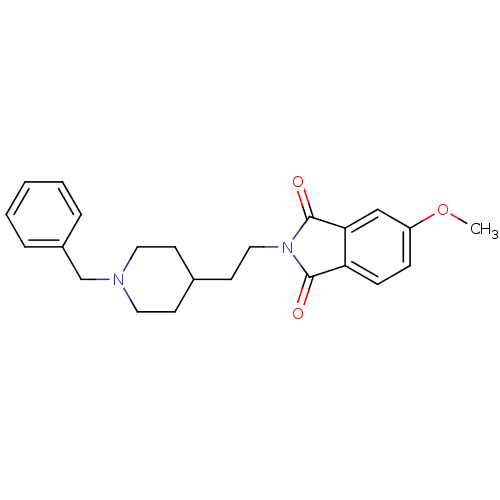 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-5-methoxyisoin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50004000 ((3aS,8aR)-1,3a,8-trimethyl-1,2,3,3a,8,8a-hexahydro...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against butyrylcholinesterase (BuChE) obtained from rat plasma | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003998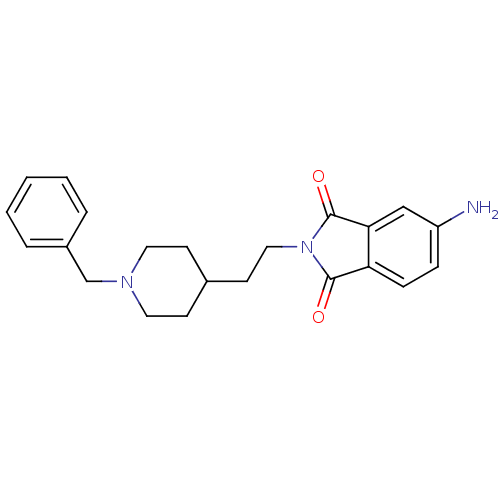 (5-Amino-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-isoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004009 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-4-nitroisoindo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004015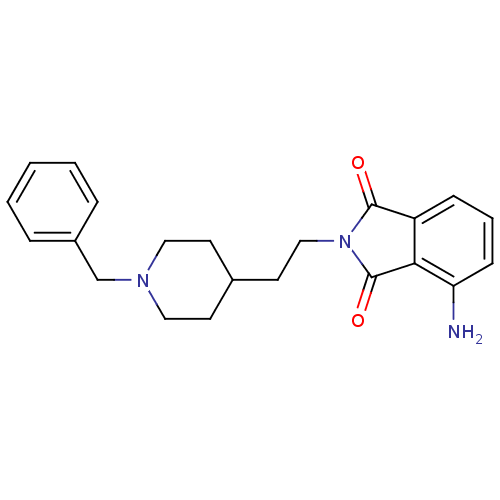 (4-Amino-2-[2-(1-benzyl-piperidin-4-yl)-ethyl]-isoi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004013 (6-(2-(1-benzylpiperidin-4-yl)ethyl)-5H-pyrrolo[3,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004040 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-5-nitroisoindo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004033 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-3,4-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004026 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-1,2-dihydroiso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004037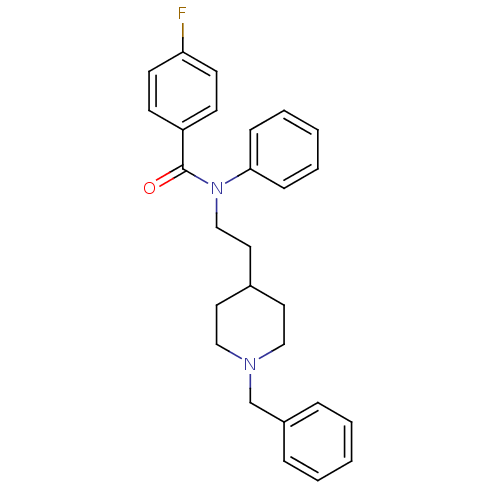 (CHEMBL140737 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004012 (2-(2-(1-benzylpiperidin-4-yl)ethyl)isoquinoline-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004020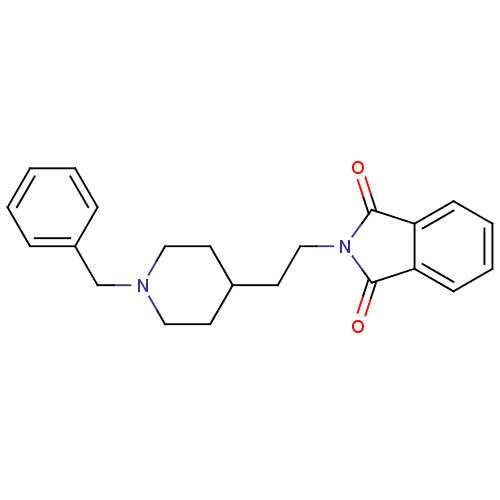 (2-(2-(1-benzylpiperidin-4-yl)ethyl)isoindoline-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9408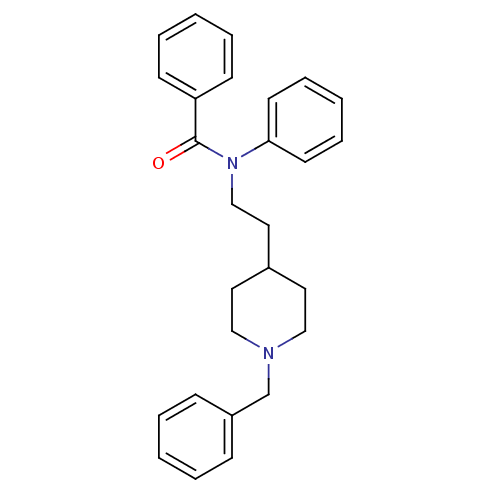 ((2E)-but-2-enedioic acid; N-[2-(1-benzylpiperidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004017 (CHEMBL127527 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004027 (CHEMBL340672 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004036 (CHEMBL340621 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004028 (CHEMBL141167 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against Butyrylcholinesterase obtained from rat plasma | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004022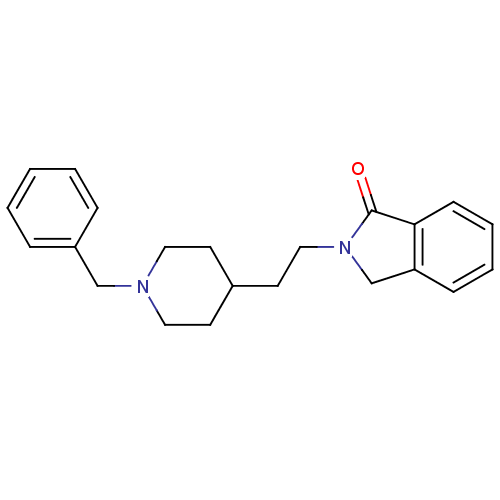 (2-(2-(1-benzylpiperidin-4-yl)ethyl)isoindolin-1-on...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004030 (CHEMBL141352 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9405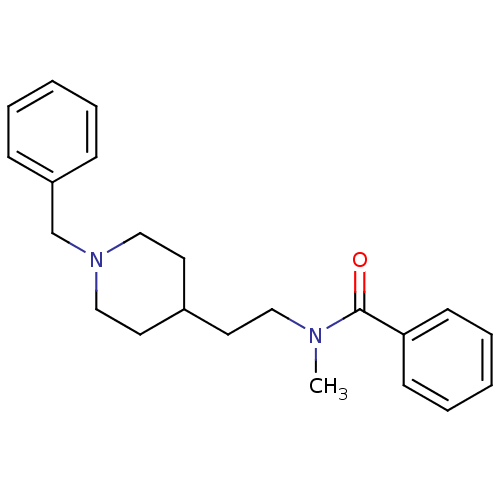 (CHEMBL56470 | N-[2-(1-Benzyl-piperidin-4-yl)-ethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004002 (CHEMBL340609 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004041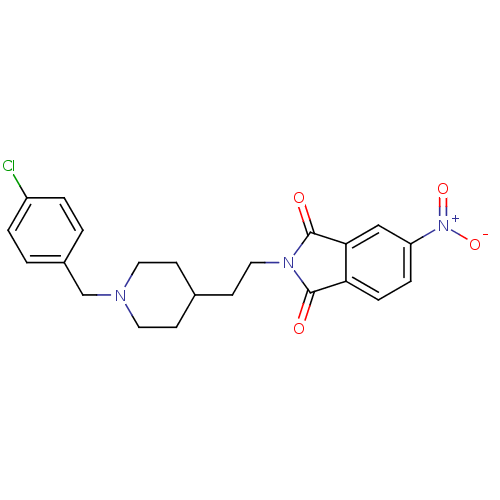 (2-(2-(1-(4-chlorobenzyl)piperidin-4-yl)ethyl)-5-ni...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004014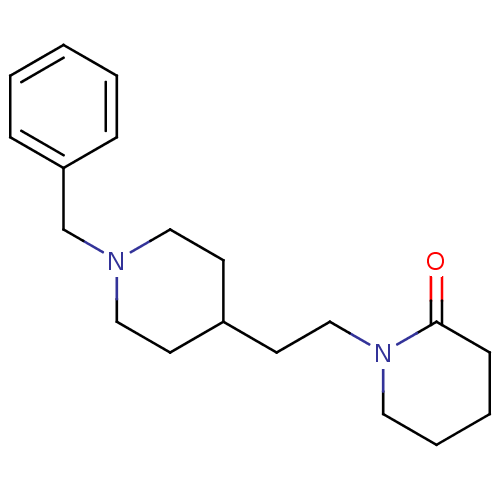 (1-(2-(1-benzylpiperidin-4-yl)ethyl)piperidin-2-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004034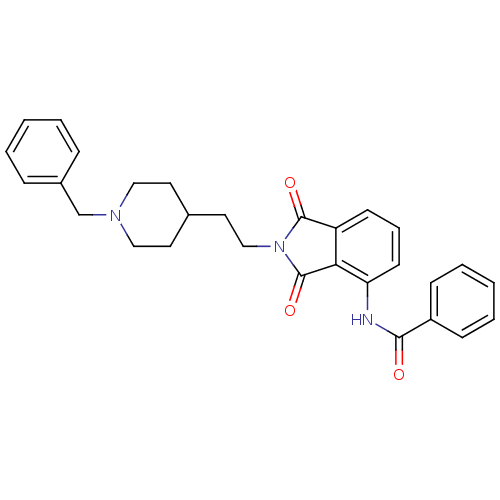 (CHEMBL141622 | N-(2-(2-(1-benzylpiperidin-4-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004042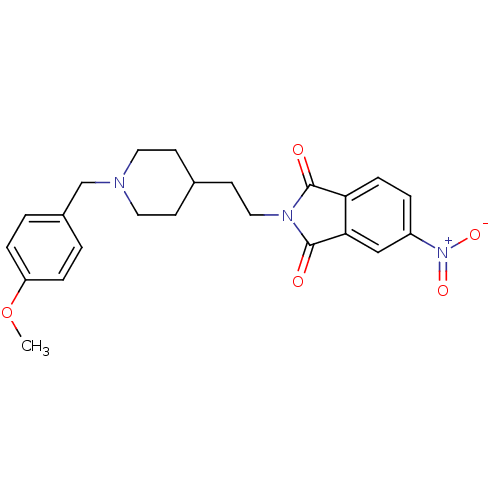 (2-(2-(1-(4-methoxybenzyl)piperidin-4-yl)ethyl)-5-n...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM9385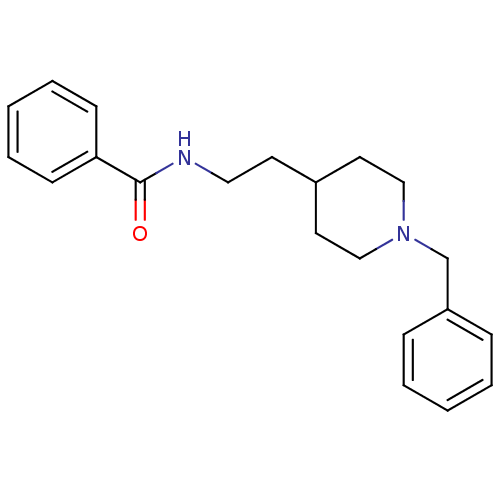 (1-Benzyl-4-[2-(N-benzoylamino)ethyl]piperidine Hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004006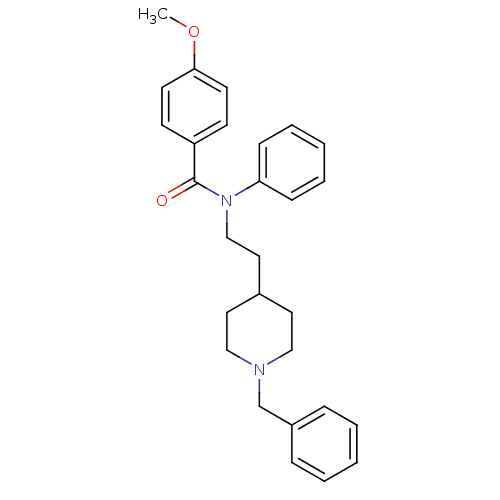 (CHEMBL141873 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004021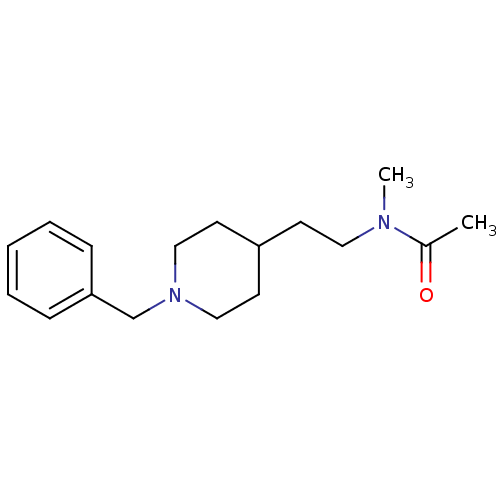 (CHEMBL125578 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50003997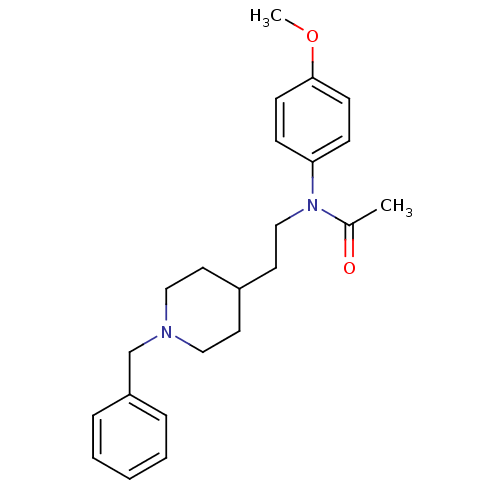 (CHEMBL141801 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004008 (3-(2-(1-benzylpiperidin-4-yl)ethyl)-2,3-dihydroqui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004005 (CHEMBL141853 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004003 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-3,4-dihydroiso...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004038 (2-(2-(1-benzylpiperidin-4-yl)ethyl)isoquinolin-1(2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004032 (3-(2-(1-benzylpiperidin-4-yl)ethyl)quinazolin-4(3H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004029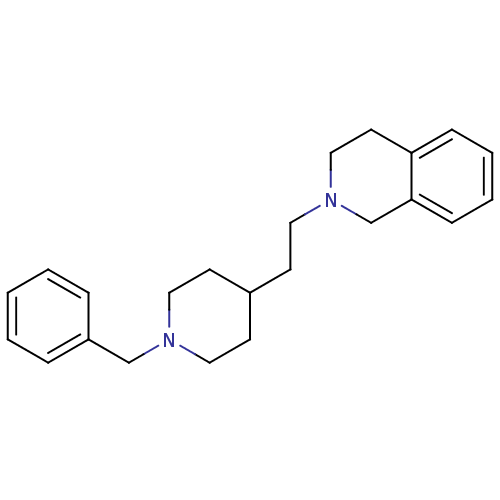 (2-(2-(1-benzylpiperidin-4-yl)ethyl)-1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004010 (2-((1-benzylpiperidin-4-yl)methyl)-5-nitroisoindol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004023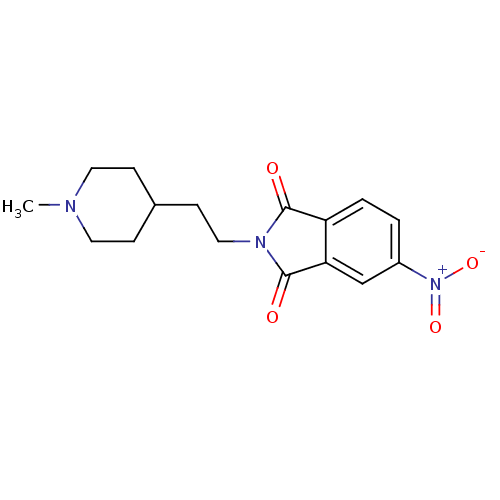 (2-(2-(1-methylpiperidin-4-yl)ethyl)-5-nitroisoindo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004019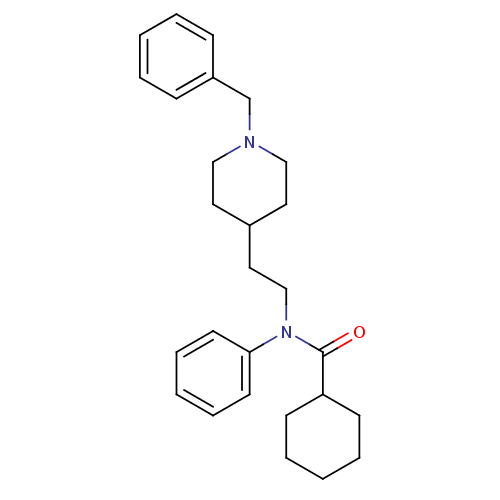 (CHEMBL140769 | Cyclohexanecarboxylic acid [2-(1-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM9409 (CHEMBL299708 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against Butyrylcholinesterase obtained from rat plasma | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004024 (CHEMBL140936 | N-(2-(1-benzylpiperidin-4-yl)ethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Mus musculus (mouse)) | BDBM50004031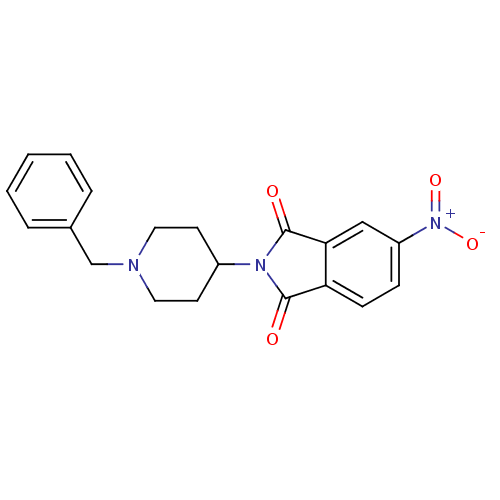 (2-(1-Benzyl-piperidin-4-yl)-5-nitro-isoindole-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration against acetylcholinesterase (AChE) obtained from mouse brain homogenate. | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50004016 (CHEMBL138107 | CHEMBL544159 | N-(2-(2-(1-benzylpip...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description In vitro inhibitory concentration of the compound against Butyrylcholinesterase obtained from rat plasma | J Med Chem 35: 4542-8 (1993) BindingDB Entry DOI: 10.7270/Q25M64P8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||