

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor B (RAT) | BDBM50004154 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004155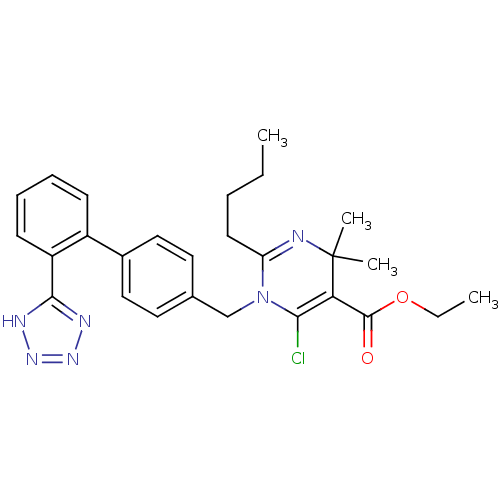 (2-Butyl-6-chloro-4,4-dimethyl-1-[2'-(1H-tetrazol-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004164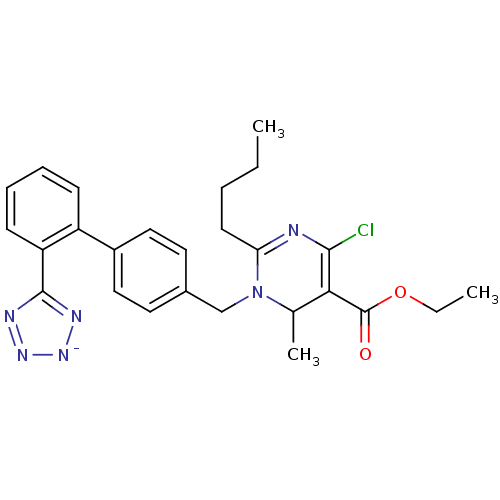 (2-Butyl-4-chloro-6-methyl-1-[2'-(1H-tetrazol-5-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50406795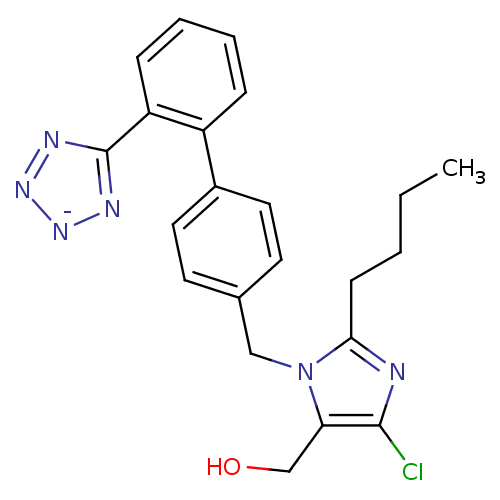 (Cozaar | LOSARTAN POTASSIUM) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004153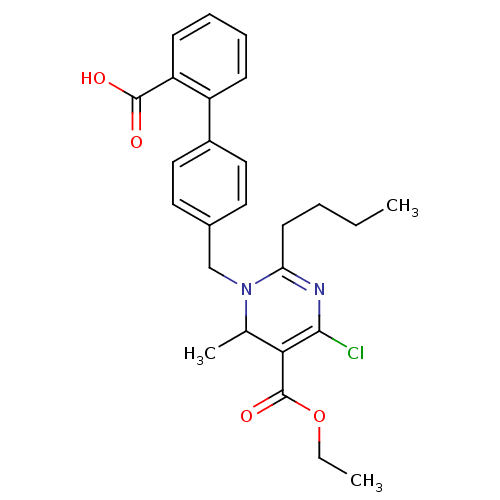 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004160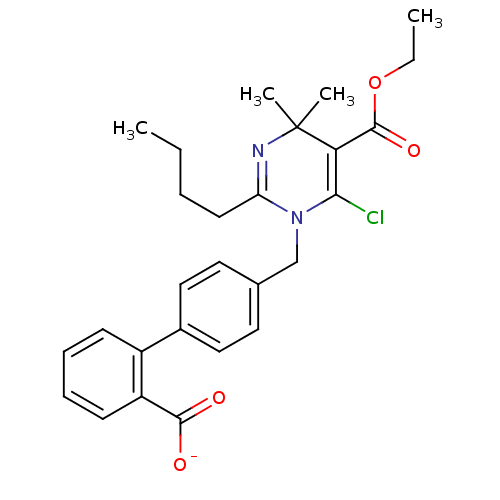 (CHEMBL145036 | Sodium; 4'-(2-butyl-6-chloro-5-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004163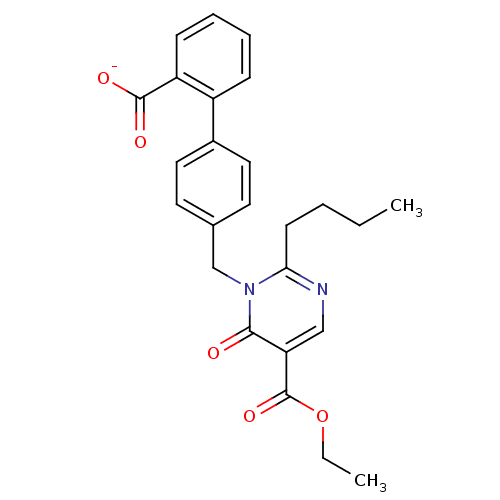 (CHEMBL342287 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM82258 (CAS_114798-26-4 | Losartan | NSC_3961) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro antagonistic potency against angiotensin II receptor using [125I]- Sar,Ile8-angiotensin II as the radioligand in rat adrenal cortical membra... | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004162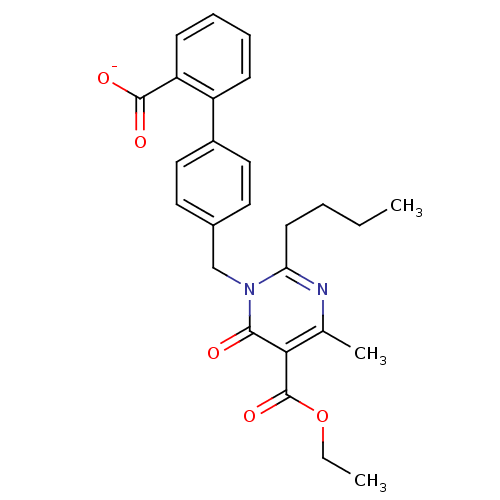 (CHEMBL145276 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004156 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4-(4-ch...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004157 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-6-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004158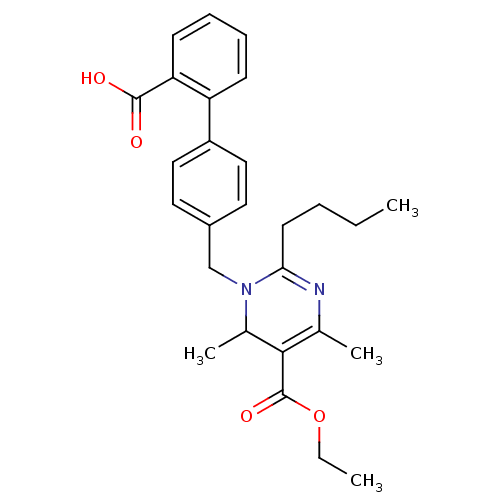 (2-Butyl-1-(2'-carboxy-biphenyl-4-ylmethyl)-4,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004159 (CHEMBL343622 | Sodium; 4'-(2-butyl-4-chloro-5-etho...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor B (RAT) | BDBM50004165 (CHEMBL144952 | Sodium; 4'-(2-butyl-5-ethoxycarbony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [125I]- Sar,Ile8-angiotensin II binding to rat adrenal corticcal membrane angiotensin II receptor | J Med Chem 35: 4751-63 (1993) BindingDB Entry DOI: 10.7270/Q2VH5PFJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||